
HL Paper 2
The Haber process enables the large-scale production of ammonia needed to make fertilizers.
The equation for the Haber process is given below.
\[{{\text{N}}_2}({\text{g)}} + 3{{\text{H}}_2}({\text{g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_3}({\text{g)}}\]
The percentage of ammonia in the equilibrium mixture varies with temperature.

Ammonia can be converted into nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), and hydrocyanic acid, HCN(aq). The \({\text{p}}{K_{\text{a}}}\) of hydrocyanic acid is 9.21.
A student decided to investigate the reactions of the two acids with separate samples of \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.
(i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and explain your choice.
(ii) State and explain the effect of increasing the pressure on the yield of ammonia.
(iii) Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
(iv) A mixture of 1.00 mol \({{\text{N}}_{\text{2}}}\) and 3.00 mol \({{\text{H}}_{\text{2}}}\) was placed in a \({\text{1.0 d}}{{\text{m}}^{\text{3}}}\) flask at 400 °C. When the system was allowed to reach equilibrium, the concentration of was found to be \({\text{0.062 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the equilibrium constant, \({K_{\text{c}}}\), of the reaction at this temperature.
(v) Iron is used as a catalyst in the Haber process. State the effect of a catalyst on the value of \({K_{\text{c}}}\).
(i) Distinguish between the terms strong and weak acid and state the equations used to show the dissociation of each acid in aqueous solution.
(ii) Deduce the expression for the ionization constant, \({K_{\text{a}}}\), of hydrocyanic acid and calculate its value from the \({\text{p}}{K_{\text{a}}}\) value given.
(iii) Use your answer from part (b) (ii) to calculate the \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and the pH of an aqueous solution of hydrocyanic acid of concentration \({\text{0.108 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). State one assumption made in arriving at your answer.
A small piece of magnesium ribbon is added to solutions of nitric and hydrocyanic acid of the same concentration at the same temperature. Describe two observations that would allow you to distinguish between the two acids.
(i) Calculate the volume of the sodium hydroxide solution required to react exactly with a \({\text{15.0 c}}{{\text{m}}^{\text{3}}}\) solution of \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) nitric acid.
(ii) The following hypothesis was suggested by the student: “Since hydrocyanic acid is a weak acid it will react with a smaller volume of the \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.” Comment on whether or not this is a valid hypothesis.
(iii) Use Table 16 of the Data Booklet to identify a suitable indicator for the titration of sodium hydroxide and hydrocyanic acid.
Markscheme
(i) exothermic;
Accept either of the following for the second mark.
increasing temperature favours endothermic/reverse reaction;
as yield decreases with increasing temperature;
(ii) yield increases / equilibrium moves to the right / more ammonia;
increase in pressure favours the reaction which has fewer moles of gaseous products;
(iii) \({K_{\text{c}}} = \frac{{{{{\text{[N}}{{\text{H}}_3}{\text{]}}}^2}}}{{{\text{[}}{{\text{N}}_2}{\text{][}}{{\text{H}}_2}{{\text{]}}^3}}}\);
(iv) \({\text{[}}{{\text{N}}_2}{\text{]}}\): (at equilibrium \( = 1.00 - 0.031 = \)) \({\text{0.969 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[}}{{\text{H}}_2}{\text{]}}\): (at equilibrium \( = 3.00 - 3(0.031) = \)) \({\text{2.91 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({K_{\text{c}}}{\text{ }}\left( { = \frac{{{{{\text{(0.062)}}}^2}}}{{{\text{(0.969) (2.91}}{{\text{)}}^3}}}} \right) = {\text{1.6(1)}} \times {\text{1}}{{\text{0}}^{ - 4}}\);
Ignore units.
Award [1] for Kc = 1.4 \( \times \) 10–4
(v) no effect;
(i) strong acid completely dissociated/ionized and weak acid partially dissociated/ionized;
\({\text{HN}}{{\text{O}}_3}{\text{(aq)}} \to {{\text{H}}^ + }{\text{(aq)}} + {\text{NO}}_3^ - {\text{(aq)}}\);
\({\text{HCN(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{N}}^ - }{\text{(aq)}}\);
Insist on both arrows as shown.
State symbols not needed.
Accept H2O and H3O+.
(ii) \({K_{\text{a}}} = \frac{{{\text{[}}{{\text{H}}^ + }{\text{][C}}{{\text{N}}^ - }{\text{]}}}}{{{\text{[HCN]}}}}\);
Allow H3O+ instead of H+.
\({K_{\text{a}}} = {10^{ - 9.21}} = 6.17 \times {10^{ - 10}}\);
(iii) \({[{{\text{H}}^ + }] = \sqrt {{K_{\text{a}}}[{\text{HCN}}]} /\sqrt {(6.17 \times {{10}^{ - 10}} \times 0.108)} }\);
\({ = 8.16 \times {{10}^{ - 6}}}\);
Allow in the range 8.13 \( \times \) 10–6 to 8.16 \( \times \) 10–6.
\({\text{pH}} = 5.09\);
OR
\({{\text{pH}} = \frac{1}{2}{\text{(p}}{K_{\text{a}}} - {\text{log}}[{\text{HCN}}])/\frac{1}{2}(9.21 - \log \,0.108)}\);
\({ = 5.09}\);
\({\text{[}}{{\text{H}}^ + }{\text{]}} = {10^{ - 5.09}} = 8.16 \times {10^{ - 6}}\);
Allow in the range 8.13 \( \times \) 10–6 to 8.16 \( \times \) 10–6.
If expression for [H+] missing but both answers correct, award [3], if one answer
correct, award [2].
assume \({\text{[}}{{\text{H}}^ + }{\text{]}} \ll 0.108\) / negligible dissociation;
With HNO3:
faster rate of bubble/hydrogen/gas production;
faster rate of magnesium dissolving;
higher temperature change;
Accept opposite argument for HCN.
Reference to specific observations needed.
Award [1] if 2 observations given but acid is not identified.
(i) (nitric acid) 7.5 cm3;
(ii) not valid as hydrocyanic acid reacts with same volume/ 7.5 cm3;
(iii) bromothymol blue / phenol red / phenolphthalein;
Examiners report
Equilibrium is a topic that has shown substantial improvement in recent sessions with some very well produced arguments. The reaction was correctly described as exothermic with a reason correctly given in most cases. Most candidates knew that yield would increase with increased pressure, but some failed to identify the change in the number of “gaseous” molecules as the reason. More candidates had difficulty with the equilibrium constant calculation often using the initial not equilibrium concentrations.
In (b) most correctly defined strong and weak acids and many also wrote correct equations. A few, however, missed the equilibrium sign for hydrocyanic acid. HA, CH3COOH and HCl were commonly given instead of HCN and HNO3, suggesting that students sometimes have difficulty applying general concepts to specific cases. It was encouraging to see many candidates determine the pH from the pKa value including the assumption that there is negligible dissociation, as this has challenged students in previous sessions. A significant number of weaker candidates reported however that the acid solution would have pH values above 7.
Part (c) presented problems with many candidates unable to describe specific observations related to rate which would distinguish between a strong and weak acid and simply stated that the reaction would be faster.
The moles calculation was answered well in (d) with most candidates able to identify phenolphthalein as a suitable indicator.
Bleaches in which chlorine is the active ingredient are the most common, although some environmental groups have concerns about their use.
In aqueous chlorine the equilibrium below produces chloric(I) acid (hypochlorous acid), HOCl, the active bleach.
\[{\text{C}}{{\text{l}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HOCl(aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\]
Aqueous sodium chlorate(I), NaOCl, the most common active ingredient in chlorine based bleaches, oxidizes coloured materials to colourless products while being reduced to the chloride ion. It will also oxidize sulfur dioxide to the sulfate ion.
The standard electrode potential for the reduction of the chlorate(V) ion to the chloride ion is \( + 1.49{\text{ V}}\).
(i) Describe the colour change that occurs when aqueous chlorine is added to aqueous sodium bromide.
(ii) Outline, with the help of a chemical equation, why this reaction occurs.
Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is indicated in the equation above.
State a balanced equation for the reaction of chloric(I) acid with water.
Outline, in terms of the equilibrium in aqueous chlorine, why it is dangerous to use an acidic toilet cleaner in combination with this kind of bleach.
Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
Partial neutralization of chloric(I) acid creates a buffer solution. Given that the \({\text{p}}{K_{\text{a}}}\) of chloric(I) acid is 7.53, determine the pH of a solution that has \({\text{[HOCl]}} = 0.100{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{[Cl}}{{\text{O}}^ - }{\text{]}} = 0.0500{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
Describe, using HIn to represent the indicator in its acid form, why an indicator changes colour when excess alkali is added.
(i) Deduce a balanced equation for the reaction between the chlorate(I) ion and sulfur dioxide from the appropriate half-equations.
(ii) State the initial and final oxidation numbers of both chlorine and sulfur in the final equation.

(i) Define the term standard electrode potential.
(ii) Referring to Table 14 of the Data Booklet, deduce, giving a reason, whether the oxidation of the chromium(III) ion to the dichromate(VI) ion by the chlorate(V) ion is energetically feasible.
Markscheme
(i) from (pale) green/colourless to yellow/orange/brown;
Initial colour must be stated.
Do not accept “clear/transparent” instead of “colourless”.
(ii) chlorine more reactive/more powerful oxidizing agent (than bromine);
Accept opposite statements for bromine.
Accept “chloride ion a weaker reducing agent” / “bromide ion a stronger reducing agent”.
Accept “chlorine more electronegative than bromine”.
\({\text{C}}{{\text{l}}_2}{\text{(aq)}} + {\text{2NaBr(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2NaCl(aq)}}/{\text{C}}{{\text{l}}_2}{\text{(aq)}} + {\text{2B}}{{\text{r}}^ - }{\text{(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2C}}{{\text{l}}^ - }{\text{(aq)}}\);
Ignore state symbols.
Do not accept with equilibrium sign.
chloric(I) acid (shown as) a molecule/molecular, but hydrochloric acid (shown as being) split into ions / OWTTE;
Accept “chloric(I) acid is partially dissociated and hydrochloric acid is fully dissociated”.
Reference needed to both acids for mark.
\({\text{HOCl(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}}/{\text{HOCl(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}}\);
Equilibrium sign required for the mark.
Ignore state symbols.
acid displaces the equilibrium to the left (to form chlorine);
chlorine is toxic/poisonous/harmful/lung irritant;
Accept answers that refer to the (b) (ii) equilibrium.
chloric(I) acid has –OH group / hydrogen attached to a very electronegative atom;
Accept polar molecule.
can form hydrogen bonds to water;
hydrogen bonding to water increases its solubility;
(as a weak acid it is) in equilibrium with ions;
\({K_{\text{a}}} = {10^{ - 7.53}} = 2.95 \times {10^{ - 8}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}})\);
\({K_{\text{a}}} = \frac{{{\text{[}}{{\text{H}}^ + }{\text{][Cl}}{{\text{O}}^ - }{\text{]}}}}{{{\text{[HOCl]}}}} = \frac{{{\text{[}}{{\text{H}}^ + }{\text{](0.05)}}}}{{{\text{(0.1)}}}} \approx \frac{{{\text{[}}{{\text{H}}^ + }{\text{]}}}}{2} = {\text{2.95}} \times {\text{1}}{{\text{0}}^{ - 8}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[}}{{\text{H}}^ + }{\text{]}} = 2 \times 2.95 \times {10^{ - 8}} = 5.9 \times {10^{ - 8}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{pH}} = - \log (5.9 \times {10^{ - 8}}) = 7.23\);
Accept other methods of carrying out the calculation.
Award [4] for correct final answer.
\({\text{HIn}} \rightleftharpoons {{\text{H}}^ + } + {\text{I}}{{\text{n}}^ - }\);
Do not accept equation without equilibrium arrow.
(weak acid in which the) acid/HIn and conjugate base/In– have different colours / OWTTE;
excess alkali shifts the equilibrium to the RHS/towards the conjugate base;
(i) \({\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\);
\({\text{SO}}_4^{2 - }{\text{(aq)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{S}}{{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\);
Accept SO42–(aq) + 4H+(aq) + 2e– \( \rightleftharpoons \) H2SO3(aq) + H2O(l).
For final equation:
\({\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} + {\text{S}}{{\text{O}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{SO}}_4^{2 - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\)
Accept ClO–(aq) + H2SO3(aq) \( \rightleftharpoons \) SO42–(aq) + 2H+(aq) + Cl–(aq).
correct reactants and products;
balancing and cancelling \({{\text{e}}^ - }\), \({{\text{H}}^ + }\) and \({{\text{H}}_2}{\text{O}}\);
Apply ECF if incorrect half-equations written.
Ignore state symbols and absence of equilibrium arrow for all equations and accept inclusion of Na+ in any equation.
(ii) Award [2] for all correct, [1] for 2 or 3 correct.

Remember to apply ECF from final (c) (i) equation.
Penalise incorrect notation (eg, 4 or 4+ rather than +4) once only, so award [1] for a fully correct answer in an incorrect format.
(i) potential (of reduction half-reaction) under standard conditions measured
relative to standard hydrogen electrode/SHE / OWTTE;
Allow “solute concentration of 1 mol dm–3” or “1 bar/1 atm (pressure) for gases” instead of “standard conditions”.
(ii) yes / energetically feasible;
would have a positive \({E_{{\text{cell}}}}\) / chlorate(V) ion stronger oxidizing agent than dichromate(VI) ion / OWTTE;
Examiners report
Though it was the least popular question on the paper, it was still answered, though not very well, by a significant number of students. Correct responses to the colour change required in the first part were rare, though more students could write an appropriate equation and outline why the reaction occurred, even though this was often phrased in terms of electronegativity, rather than reactivity or electrode potential. In part (b) many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer the question asked and frequently, even having displayed this knowledge, were unable to write an equation for the required reaction in water. Unfortunately changes in pagination meant that the phrase “the equilibrium above” in part (b) (iii) may have confused candidates with regard to which particular equilibrium the question referred to. Fortunately both of the equilibria that it could have referred to change in the same direction and students scored well on this, and both would eventually result in the release of chlorine, that a number recognized as a toxic gas. In contrast to Question 1, many students could correctly identify the hydrogen bonding, resulting from the –OH group, as being the reason for the solubility of HOCl in water. An encouraging number of students gained full marks for calculating the pH of the buffer, usually by memorizing the Henderson-Hasselbalch equation and substituting in this. An even greater number of students could accurately explain the mode of action of acid-base indicators. In part (c) very few students could write, much less combine, appropriate half equations, even though the reactants and products were given, but far more could correctly deduce the oxidation numbers of the species involved. In the final part most students had some general idea of what a standard electrode potential was, but in many cases the definitions lacked the detail required. Quite a few students correctly deduced that the oxidation of chromium(III) to dichromate(VI) was energetically feasible and give valid reasons to support this.
Though it was the least popular question on the paper, it was still answered, though not very well, by a significant number of students. Correct responses to the colour change required in the first part were rare, though more students could write an appropriate equation and outline why the reaction occurred, even though this was often phrased in terms of electronegativity, rather than reactivity or electrode potential. In part (b) many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer the question asked and frequently, even having displayed this knowledge, were unable to write an equation for the required reaction in water. Unfortunately changes in pagination meant that the phrase “the equilibrium above” in part (b) (iii) may have confused candidates with regard to which particular equilibrium the question referred to. Fortunately both of the equilibria that it could have referred to change in the same direction and students scored well on this, and both would eventually result in the release of chlorine, that a number recognized as a toxic gas. In contrast to Question 1, many students could correctly identify the hydrogen bonding, resulting from the –OH group, as being the reason for the solubility of HOCl in water. An encouraging number of students gained full marks for calculating the pH of the buffer, usually by memorizing the Henderson-Hasselbalch equation and substituting in this. An even greater number of students could accurately explain the mode of action of acid-base indicators. In part (c) very few students could write, much less combine, appropriate half equations, even though the reactants and products were given, but far more could correctly deduce the oxidation numbers of the species involved. In the final part most students had some general idea of what a standard electrode potential was, but in many cases the definitions lacked the detail required. Quite a few students correctly deduced that the oxidation of chromium(III) to dichromate(VI) was energetically feasible and give valid reasons to support this.
Though it was the least popular question on the paper, it was still answered, though not very well, by a significant number of students. Correct responses to the colour change required in the first part were rare, though more students could write an appropriate equation and outline why the reaction occurred, even though this was often phrased in terms of electronegativity, rather than reactivity or electrode potential. In part (b) many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer the question asked and frequently, even having displayed this knowledge, were unable to write an equation for the required reaction in water. Unfortunately changes in pagination meant that the phrase “the equilibrium above” in part (b) (iii) may have confused candidates with regard to which particular equilibrium the question referred to. Fortunately both of the equilibria that it could have referred to change in the same direction and students scored well on this, and both would eventually result in the release of chlorine, that a number recognized as a toxic gas. In contrast to Question 1, many students could correctly identify the hydrogen bonding, resulting from the –OH group, as being the reason for the solubility of HOCl in water. An encouraging number of students gained full marks for calculating the pH of the buffer, usually by memorizing the Henderson-Hasselbalch equation and substituting in this. An even greater number of students could accurately explain the mode of action of acid-base indicators. In part (c) very few students could write, much less combine, appropriate half equations, even though the reactants and products were given, but far more could correctly deduce the oxidation numbers of the species involved. In the final part most students had some general idea of what a standard electrode potential was, but in many cases the definitions lacked the detail required. Quite a few students correctly deduced that the oxidation of chromium(III) to dichromate(VI) was energetically feasible and give valid reasons to support this.
Though it was the least popular question on the paper, it was still answered, though not very well, by a significant number of students. Correct responses to the colour change required in the first part were rare, though more students could write an appropriate equation and outline why the reaction occurred, even though this was often phrased in terms of electronegativity, rather than reactivity or electrode potential. In part (b) many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer the question asked and frequently, even having displayed this knowledge, were unable to write an equation for the required reaction in water. Unfortunately changes in pagination meant that the phrase “the equilibrium above” in part (b) (iii) may have confused candidates with regard to which particular equilibrium the question referred to. Fortunately both of the equilibria that it could have referred to change in the same direction and students scored well on this, and both would eventually result in the release of chlorine, that a number recognized as a toxic gas. In contrast to Question 1, many students could correctly identify the hydrogen bonding, resulting from the –OH group, as being the reason for the solubility of HOCl in water. An encouraging number of students gained full marks for calculating the pH of the buffer, usually by memorizing the Henderson-Hasselbalch equation and substituting in this. An even greater number of students could accurately explain the mode of action of acid-base indicators. In part (c) very few students could write, much less combine, appropriate half equations, even though the reactants and products were given, but far more could correctly deduce the oxidation numbers of the species involved. In the final part most students had some general idea of what a standard electrode potential was, but in many cases the definitions lacked the detail required. Quite a few students correctly deduced that the oxidation of chromium(III) to dichromate(VI) was energetically feasible and give valid reasons to support this.
Though it was the least popular question on the paper, it was still answered, though not very well, by a significant number of students. Correct responses to the colour change required in the first part were rare, though more students could write an appropriate equation and outline why the reaction occurred, even though this was often phrased in terms of electronegativity, rather than reactivity or electrode potential. In part (b) many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer the question asked and frequently, even having displayed this knowledge, were unable to write an equation for the required reaction in water. Unfortunately changes in pagination meant that the phrase “the equilibrium above” in part (b) (iii) may have confused candidates with regard to which particular equilibrium the question referred to. Fortunately both of the equilibria that it could have referred to change in the same direction and students scored well on this, and both would eventually result in the release of chlorine, that a number recognized as a toxic gas. In contrast to Question 1, many students could correctly identify the hydrogen bonding, resulting from the –OH group, as being the reason for the solubility of HOCl in water. An encouraging number of students gained full marks for calculating the pH of the buffer, usually by memorizing the Henderson-Hasselbalch equation and substituting in this. An even greater number of students could accurately explain the mode of action of acid-base indicators. In part (c) very few students could write, much less combine, appropriate half equations, even though the reactants and products were given, but far more could correctly deduce the oxidation numbers of the species involved. In the final part most students had some general idea of what a standard electrode potential was, but in many cases the definitions lacked the detail required. Quite a few students correctly deduced that the oxidation of chromium(III) to dichromate(VI) was energetically feasible and give valid reasons to support this.
Though it was the least popular question on the paper, it was still answered, though not very well, by a significant number of students. Correct responses to the colour change required in the first part were rare, though more students could write an appropriate equation and outline why the reaction occurred, even though this was often phrased in terms of electronegativity, rather than reactivity or electrode potential. In part (b) many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer the question asked and frequently, even having displayed this knowledge, were unable to write an equation for the required reaction in water. Unfortunately changes in pagination meant that the phrase “the equilibrium above” in part (b) (iii) may have confused candidates with regard to which particular equilibrium the question referred to. Fortunately both of the equilibria that it could have referred to change in the same direction and students scored well on this, and both would eventually result in the release of chlorine, that a number recognized as a toxic gas. In contrast to Question 1, many students could correctly identify the hydrogen bonding, resulting from the –OH group, as being the reason for the solubility of HOCl in water. An encouraging number of students gained full marks for calculating the pH of the buffer, usually by memorizing the Henderson-Hasselbalch equation and substituting in this. An even greater number of students could accurately explain the mode of action of acid-base indicators. In part (c) very few students could write, much less combine, appropriate half equations, even though the reactants and products were given, but far more could correctly deduce the oxidation numbers of the species involved. In the final part most students had some general idea of what a standard electrode potential was, but in many cases the definitions lacked the detail required. Quite a few students correctly deduced that the oxidation of chromium(III) to dichromate(VI) was energetically feasible and give valid reasons to support this.
Though it was the least popular question on the paper, it was still answered, though not very well, by a significant number of students. Correct responses to the colour change required in the first part were rare, though more students could write an appropriate equation and outline why the reaction occurred, even though this was often phrased in terms of electronegativity, rather than reactivity or electrode potential. In part (b) many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer the question asked and frequently, even having displayed this knowledge, were unable to write an equation for the required reaction in water. Unfortunately changes in pagination meant that the phrase “the equilibrium above” in part (b) (iii) may have confused candidates with regard to which particular equilibrium the question referred to. Fortunately both of the equilibria that it could have referred to change in the same direction and students scored well on this, and both would eventually result in the release of chlorine, that a number recognized as a toxic gas. In contrast to Question 1, many students could correctly identify the hydrogen bonding, resulting from the –OH group, as being the reason for the solubility of HOCl in water. An encouraging number of students gained full marks for calculating the pH of the buffer, usually by memorizing the Henderson-Hasselbalch equation and substituting in this. An even greater number of students could accurately explain the mode of action of acid-base indicators. In part (c) very few students could write, much less combine, appropriate half equations, even though the reactants and products were given, but far more could correctly deduce the oxidation numbers of the species involved. In the final part most students had some general idea of what a standard electrode potential was, but in many cases the definitions lacked the detail required. Quite a few students correctly deduced that the oxidation of chromium(III) to dichromate(VI) was energetically feasible and give valid reasons to support this.
Though it was the least popular question on the paper, it was still answered, though not very well, by a significant number of students. Correct responses to the colour change required in the first part were rare, though more students could write an appropriate equation and outline why the reaction occurred, even though this was often phrased in terms of electronegativity, rather than reactivity or electrode potential. In part (b) many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer the question asked and frequently, even having displayed this knowledge, were unable to write an equation for the required reaction in water. Unfortunately changes in pagination meant that the phrase “the equilibrium above” in part (b) (iii) may have confused candidates with regard to which particular equilibrium the question referred to. Fortunately both of the equilibria that it could have referred to change in the same direction and students scored well on this, and both would eventually result in the release of chlorine, that a number recognized as a toxic gas. In contrast to Question 1, many students could correctly identify the hydrogen bonding, resulting from the –OH group, as being the reason for the solubility of HOCl in water. An encouraging number of students gained full marks for calculating the pH of the buffer, usually by memorizing the Henderson-Hasselbalch equation and substituting in this. An even greater number of students could accurately explain the mode of action of acid-base indicators. In part (c) very few students could write, much less combine, appropriate half equations, even though the reactants and products were given, but far more could correctly deduce the oxidation numbers of the species involved. In the final part most students had some general idea of what a standard electrode potential was, but in many cases the definitions lacked the detail required. Quite a few students correctly deduced that the oxidation of chromium(III) to dichromate(VI) was energetically feasible and give valid reasons to support this.
Though it was the least popular question on the paper, it was still answered, though not very well, by a significant number of students. Correct responses to the colour change required in the first part were rare, though more students could write an appropriate equation and outline why the reaction occurred, even though this was often phrased in terms of electronegativity, rather than reactivity or electrode potential. In part (b) many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer the question asked and frequently, even having displayed this knowledge, were unable to write an equation for the required reaction in water. Unfortunately changes in pagination meant that the phrase “the equilibrium above” in part (b) (iii) may have confused candidates with regard to which particular equilibrium the question referred to. Fortunately both of the equilibria that it could have referred to change in the same direction and students scored well on this, and both would eventually result in the release of chlorine, that a number recognized as a toxic gas. In contrast to Question 1, many students could correctly identify the hydrogen bonding, resulting from the –OH group, as being the reason for the solubility of HOCl in water. An encouraging number of students gained full marks for calculating the pH of the buffer, usually by memorizing the Henderson-Hasselbalch equation and substituting in this. An even greater number of students could accurately explain the mode of action of acid-base indicators. In part (c) very few students could write, much less combine, appropriate half equations, even though the reactants and products were given, but far more could correctly deduce the oxidation numbers of the species involved. In the final part most students had some general idea of what a standard electrode potential was, but in many cases the definitions lacked the detail required. Quite a few students correctly deduced that the oxidation of chromium(III) to dichromate(VI) was energetically feasible and give valid reasons to support this.
A class studied the equilibrium established when ethanoic acid and ethanol react together in the presence of a strong acid, using propanone as an inert solvent. The equation is given below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
One group made the following initial mixture:

After one week, a \(5.00 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\) sample of the final equilibrium mixture was pipetted out and titrated with \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide to determine the amount of ethanoic acid remaining. The following titration results were obtained:

The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine the amount, in mol, of ethanoic acid present in the initial mixture.
The concentration of ethanoic acid can be calculated as \({\text{1.748 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the percentage uncertainty of this value. (Neglect any uncertainty in the density and the molar mass.)
Calculate the absolute uncertainty of the titre for Titration 1 (\({\text{27.60 c}}{{\text{m}}^3}\)).
Suggest the average volume of alkali, required to neutralize the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample, that the student should use.
\({\text{3.00 c}}{{\text{m}}^{\text{3}}}\) of the \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide reacted with the hydrochloric acid present in the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample. Determine the concentration of ethanoic acid in the final equilibrium mixture.
Deduce the equilibrium constant expression for the reaction.
The other concentrations in the equilibrium mixture were calculated as follows:

Use these data, along with your answer to part (iii), to determine the value of the equilibrium constant. (If you did not obtain an answer to part (iii), assume the concentrations of ethanol and ethanoic acid are equal, although this is not the case.)
Outline how you could establish that the system had reached equilibrium at the end of one week.
Outline why changing the temperature has only a very small effect on the value of the equilibrium constant for this equilibrium.
Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of ethanoic acid converted to product.
Propanone is used as the solvent because one compound involved in the equilibrium is insoluble in water. Identify this compound and explain why it is insoluble in water.
Suggest one other reason why using water as a solvent would make the experiment less successful.
Markscheme
\({\text{M(C}}{{\text{H}}_{\text{3}}}{\text{COOH)}}\left( { = (4 \times 1.01) + (2 \times 12.01) + (2 \times 16.00)} \right) = 60.06{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Accept 60 (g mol–1).
\({\text{mass (C}}{{\text{H}}_3}{\text{COOH) }}( = 5.00 \times 1.05) = 5.25{\text{ (g)}}\);
\(\frac{{5.25}}{{60.06}} = 0.0874{\text{ (mol)}}\);
Award [3] for correct final answer.
Accept 0.0875 (comes from using Mr = 60 g mol–1).
percentage uncertainty in volume of ethanoic acid \( = 100 \times \frac{{0.05}}{{5.00}}{\text{ }} = 1\% \);
percentage uncertainty in total volume \( = 100 \times \frac{{0.62}}{{50}} = 1.24\% \);
total percentage uncertainty \( = 1 + 1.24 = 2.24\% \);
Accept rounding down to 2.2/2%.
\( \pm 0.1/0.10{\text{ }}({\text{c}}{{\text{m}}^3})\);
Do not accept without ±.
\({\text{26.00 (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
\(26.00 - 3.00 = 23.00{\text{ }}({\text{c}}{{\text{m}}^3})\);
If other methods used, award M1 for calculating amount of NaOH reacting with CH3COOH.
\(0.200 \times \frac{{23.00}}{{5.00}} = 0.920{\text{ }}({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}})\);
Award [2] for correct final answer.
If (ii) given as mean titre (26.5 cm3) then ECF answer comes to 0.94 (mol dm–3).
\(({K_{\text{c}}} = )\frac{{{\text{[C}}{{\text{H}}_3}{\text{COO}}{{\text{C}}_2}{{\text{H}}_5}{\text{][}}{{\text{H}}_2}{\text{O]}}}}{{{\text{[}}{{\text{C}}_2}{{\text{H}}_5}{\text{OH][C}}{{\text{H}}_3}{\text{COOH]}}}}\);
Do not penalize minor errors in formulas.
Accept \(({K_{\text{c}}} = )\frac{{{\text{[}}esther{\text{][}}water{\text{]}}}}{{[ethanol/alcohol{\text{][(}}ethanoic{\text{) }}acid{\text{]}}}}\).
\(({K_c} = )\frac{{0.828 \times 1.80}}{{0.884 \times 0.920}} = 1.83\);
If assumed [CH3COOH] = 0.884 mol dm-3, answer is 1.91 – allow this even if an answer was obtained for (iii).
If (ii) given as mean titre (26.5 cm3) then ECF answer comes to 1.79.
repeat the titration a day/week later (and result should be the same) / OWTTE;
Accept “concentrations/physical properties/macroscopic properties of the system do not change”.
enthalpy change/\(\Delta H\) for the reaction is (very) small / OWTTE;
decreases (the amount of ethanoic acid converted);
Accept “increases amount of ethanoic acid present at equilibrium” / OWTTE.
(adding product) shifts position of equilibrium towards reactants/LHS / increases
the rate of the reverse reaction / OWTTE;
ethyl ethanoate/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\)/ester;
forms only weak hydrogen bonds (to water);
Allow “does not hydrogen bond to water” / “hydrocarbon sections too long” / OWTTE.
M2 can only be given only if M1 correct.
(large excess of) water will shift the position of equilibrium (far to the left) / OWTTE;
Accept any other chemically sound response, such as “dissociation of ethanoic acid would affect equilibrium”.
Examiners report
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
When nitrogen gas and hydrogen gas are allowed to react in a closed container the following equilibrium is established.
\[{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g) }}\Delta H = -92.6{\text{ kJ}}\]
Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
Predict, with a reason, how each of the following changes affects the position of equilibrium.
The volume of the container is increased.
Ammonia is removed from the equilibrium mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Ammonia is manufactured by the Haber process in which iron is used as a catalyst.
Explain the effect of a catalyst on the rate of reaction.
Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in approximately 15% yield of ammonia.
(i) Explain why a temperature lower than 500 °C is not used.
(ii) Outline why a pressure higher than 200 atm is not often used.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction on page 10.
When 1.00 mol of nitrogen and 3.00 mol of hydrogen were allowed to reach equilibrium in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) container at a temperature of 500 °C and a pressure of 1000 atm, the equilibrium mixture contained 1.46 mol of ammonia.
Calculate the value of \({K_{\text{c}}}\) at 500 °C.
Define the term base according to the Lewis theory.
Define the term weak base according to the Brønsted–Lowry theory.
Deduce the formulas of conjugate acid-base pairs in the reaction below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{NH}}_{\text{3}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]

Determine the pH of a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(aq)}}\), using tables 2 and 15 of the data booklet.
(i) Sketch the pH titration curve obtained when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{N}}{{\text{H}}_{\text{3}}}{\text{(aq)}}\) is added to \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{HCl (aq)}}\).

(ii) Identify an indicator from table 16 of the data booklet that could be used for this titration.
Markscheme
rates of forward and reverse reactions are equal / opposing changes occur at equal rates;
the concentrations of all reactants and products remain constant / macroscopic properties remain constant;
closed/isolated system;
Accept “the same” for “equal” in M1 and for “constant” in M2.
The volume of the container is increased:
position of equilibrium shifts to the left/reactants and fewer moles of gas on the right hand side/pressure decreases / OWTTE;
Ammonia is removed from the equilibrium mixture:
position of equilibrium shifts to the right/products and \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) decreases so \({\text{[}}{{\text{N}}_{\text{2}}}{\text{]}}\) and \({\text{[}}{{\text{H}}_{\text{2}}}{\text{]}}\) must also decrease to keep Kc constant
OR
position of equilibrium shifts to the right/products and rate of reverse reaction decreases / OWTTE;
Award [1 max] if both predicted changes are correct.
Do not accept “to increase \([N{H_3}]\)” or reference to LCP without explanation.
minimum energy needed (by reactants/colliding particles) to react/start/initiate a reaction;
Accept “energy difference between reactants and transition state”.
more effective/successful collisions per unit time / greater proportion of collisions effective;
alternative pathway and a lower activation energy
OR
lowers activation energy so that more particles have enough energy to react;
Do not accept just “lowers/reduces the activation energy”.
Accept “provides a surface for reacting/reactants/reaction”.
(i) slower rate / OWTTE;
uneconomic / OWTTE;
(ii) high cost for building/maintaining plant / high energy cost of compressor / OWTTE;
Do not accept “high pressure is expensive” without justification.
Accept high pressure requires high energy.
\(({K_{\text{c}}} = )\frac{{{{{\text{[N}}{{\text{H}}_3}{\text{(g)]}}}^2}}}{{{\text{[}}{{\text{N}}_2}{\text{(g)]}} \times {{{\text{[}}{{\text{H}}_2}{\text{(g)]}}}^3}}}\);
Ignore state symbols.
Concentrations must be represented by square brackets.
moles at equilibrium: nitrogen 0.27, hydrogen 0.81 / concentrations at equilibrium: nitrogen \({\text{0.27 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\), hydrogen \({\text{0.81 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) (and ammonia \({\text{1.46 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\));
\({K_{\text{c}}} = 15\);
Actual calculation gives \({K_{\text{c}}}{\text{ = }}14{\text{.}}86\).
Award [2] for correct final answer.
Award [1 max] if \({K_{\text{c}}}\left( { = \frac{{{{1.46}^2}}}{{{3^3} \times 1}}} \right) = 0.079\)
electron pair donor;
Accept lone pair donor.
proton acceptor and partially/slightly ionized;
Accept “proton acceptor and partially/slightly dissociated”.

Award [1 max] for two correct acids OR two correct conjugate bases.
\({K_{\text{b}}} = \frac{{{\text{[NH}}_4^ + {\text{][O}}{{\text{H}}^ - }{\text{]}}}}{{{\text{[N}}{{\text{H}}_3}{\text{]}}}} = 1.8 \times {10^{ - 5}}/{10^{ - 4.75}}\);
\({\text{[NH}}_4^ + {\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\) and \({\text{[N}}{{\text{H}}_3}{\text{]}} \approx 1.00 \times {10^{ - 1}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[O}}{{\text{H}}^ - }{\text{]}} = (\sqrt {1.8 \times {{10}^{ - 6}}} = )1.3 \times {10^{ - 3}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}/{\text{pOH}} = 2.89\);
\({\text{pH}} = (14.0 - 2.89 = )11.1\);
Award [4] for correct final answer.
(i) 
For volume \( = 0:{\text{ pH}} = 1\);
vertical jump should be positioned in volume range \({\text{24 c}}{{\text{m}}^{\text{3}}}\) to \({\text{26 c}}{{\text{m}}^{\text{3}}}\) and include pH range between 3 to 6;
For volume = 50: pH between 8 to 11;
(ii) methyl orange / bromophenol blue / bromocresol green / methyl red;
Examiners report
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Many reactions are in a state of equilibrium.
The following reaction was allowed to reach equilibrium at 761 K.
H2 (g) + I2 (g) \( \rightleftharpoons \) 2HI (g) ΔHθ < 0
The pH of 0.010 mol dm–3 carbonic acid, H2CO3 (aq), is 4.17 at 25 °C.
H2CO3 (aq) + H2O (l) \( \rightleftharpoons \) HCO3– (aq) + H3O+ (aq).
State the equilibrium constant expression, Kc , for this reaction.
The following equilibrium concentrations in mol dm–3 were obtained at 761 K.
Calculate the value of the equilibrium constant at 761 K.
Determine the value of ΔGθ, in kJ, for the above reaction at 761 K using section 1 of the data booklet.
Calculate [H3O+] in the solution and the dissociation constant, Ka , of the acid at 25 °C.
Calculate Kb for HCO3– acting as a base.
Markscheme
Kc = \(\frac{{{{{\text{[HI]}}}^{\text{2}}}}}{{{\text{[}}{{\text{H}}_{\text{2}}}{\text{][}}{{\text{I}}_{\text{2}}}{\text{]}}}}\)
45.6
ΔGθ = «– RT ln K = – (0.00831 kJ K−1 mol−1 x 761 K x ln 45.6) =» – 24.2 «kJ»
[H3O+] = 6.76 x 10–5 «mol dm–3»
Ka = \(\frac{{{{\left( {6.76 \times {{10}^{ - 5}}} \right)}^2}}}{{\left( {0.010 - 6.76 \times {{10}^{ - 5}}} \right)}}/\frac{{{{\left( {6.76 \times {{10}^{ - 5}}} \right)}^2}}}{{0.010}}\)
4.6 x 10–7
Accept 4.57 x 10–7
Award [3] for correct final answer.
«\(\frac{{1.00 \times {{10}^{ - 14}}}}{{4.6 \times {{10}^{ - 7}}}}\) =» 2.17 x 10–8
OR
«\(\frac{{1.00 \times {{10}^{ - 14}}}}{{4.57 \times {{10}^{ - 7}}}}\) =» 2.19 x 10–8
Examiners report
A mixture of 1.00 mol SO2(g), 2.00 mol O2(g) and 1.00 mol SO3(g) is placed in a 1.00 dm3 container and allowed to reach equilibrium.
2SO2(g) + O2(g) \( \rightleftharpoons \) 2SO3(g)
Nitrogen oxide is in equilibrium with dinitrogen dioxide.
2NO(g) \( \rightleftharpoons \) N2O2(g) ΔHΘ < 0
Deduce, giving a reason, the effect of increasing the temperature on the concentration of N2O2.
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) that involves an exothermic equilibrium process.
First step: 2NO(g) \( \rightleftharpoons \) N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
The rate constant for a reaction doubles when the temperature is increased from 25.0 °C to 35 °C.
Calculate the activation energy, Ea, in kJ mol−1 for the reaction using section 1 and 2 of the data booklet.
Markscheme
[N2O2] decreases AND exothermic «thus reverse reaction favoured»
Accept “product” for [N2O2].
Do not accept just “reverse reaction favoured/shift to left” for “[N2O2] decreases”.
[1 mark]
ALTERNATIVE 1:
«from equilibrium, step 1»
\({K_c} = \frac{{{\text{[}}{{\text{N}}_2}{{\text{O}}_2}{\text{]}}}}{{{{{\text{[NO]}}}^2}}}\)
OR
[N2O2] = Kc[NO]2
«from step 2, rate «= k1[N2O2][O2] = k2K[NO]2[O2]»
rate = k[NO]2[O2]
ALTERNATIVE 2:
«from step 2» rate = k2[N2O2][O2]
«from step 1, rate(1) = k1[NO]2 = k–1[N2O2], [N2O2] = \(\frac{{{k_1}}}{{{k_{ - 1}}}}\) [NO]2»
«rate = \(\frac{{{k_1}}}{{{k_{ - 1}}}}\) k2[NO]2[O2]»
rate = k[NO]2[O2]
Award [2] for correct rate expression.
[2 marks]
«\(\ln \frac{{{k_1}}}{{{k_2}}} = \frac{{{E_a}}}{R}\left( {\frac{1}{{{T_2}}} - \frac{1}{{{T_1}}}} \right)\)»
T2 = «273 + 35 =» 308 K AND T1 = «273 + 25 =» 298 K
Ea = 52.9 «kJ mol–1»
Award [2] for correct final answer.
[2 marks]
Examiners report
Consider the following reaction studied at 263 K.
\[{\text{2NO(g)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2NOCl(g)}}\]
It was found that the forward reaction is first order with respect to \({\rm{C}}{{\rm{l}}_2}\) and second order with respect to NO. The reverse reaction is second order with respect to NOCl.
Consider the following equilibrium reaction.
\[\begin{array}{*{20}{c}} {{\text{C}}{{\text{l}}_2}({\text{g)}} + {\text{S}}{{\text{O}}_2}({\text{g)}} \rightleftharpoons {\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}({\text{g)}}}&{\Delta {H^\Theta } = - 84.5{\text{ kJ}}} \end{array}\]
In a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) closed container, at 375 °C, \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}\) and \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\) of \({\text{C}}{{\text{l}}_{\text{2}}}\) were introduced. At equilibrium, \({\text{7.65}} \times {\text{1}}{{\text{0}}^{ - 4}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) was formed.
State the rate expression for the forward reaction.
Predict the effect on the rate of the forward reaction and on the rate constant if the concentration of NO is halved.
1.0 mol of \({\rm{C}}{{\rm{l}}_2}\) and 1.0 mol of NO are mixed in a closed container at constant temperature. Sketch a graph to show how the concentration of NO and NOCl change with time until after equilibrium has been reached. Identify the point on the graph where equilibrium is established.
Consider the following reaction.
\[{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{CO(g)}} \to {\text{NO(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\]
Possible reaction mechanisms are:
\(\begin{array}{*{20}{l}} {{\text{Above 775 K:}}}&{{\text{N}}{{\text{O}}_2} + {\text{CO}} \to {\text{NO}} + {\text{C}}{{\text{O}}_{\text{2}}}}&{{\text{slow}}} \\ {{\text{Below 775 K:}}}&{{\text{2N}}{{\text{O}}_2} \to {\text{NO}} + {\text{N}}{{\text{O}}_{\text{3}}}}&{{\text{slow}}} \\ {}&{{\text{N}}{{\text{O}}_3} + {\text{CO}} \to {\text{N}}{{\text{O}}_2} + {\text{C}}{{\text{O}}_2}}&{{\text{fast}}} \end{array}\)
Based on the mechanisms, deduce the rate expressions above and below 775 K.
State two situations when the rate of a chemical reaction is equal to the rate constant.
Consider the following graph of \(\ln k\) against \(\frac{1}{T}\) for the first order decomposition of \({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}\) into \({\text{N}}{{\text{O}}_{\text{2}}}\). Determine the activation energy in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) for this reaction.
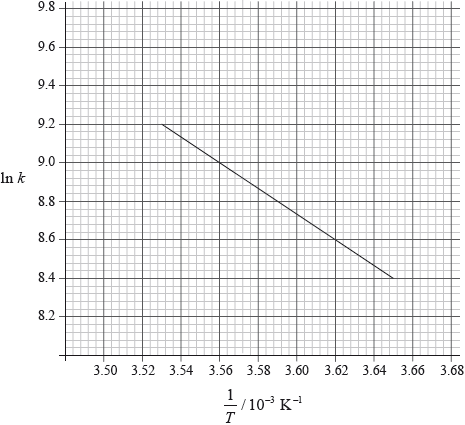
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Determine the value of the equilibrium constant, \({K_{\text{c}}}\).
If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each case, whether the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\) will increase or decrease.
If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\), predict, stating a reason in each case, how this will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}\) and the value of \({K_{\text{c}}}\).
Suggest, stating a reason, how the addition of a catalyst at constant pressure and temperature will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
Markscheme
\({\text{rate}} = k{{\text{[NO]}}^2}{\text{[C}}{{\text{l}}_{\text{2}}}{\text{]}}\);
rate of reaction will decrease by a factor of 4;
no effect on the rate constant;

y axis labelled concentration/\({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and x axis is labelled time/s;
gradient for [NO];
gradient for [NOCl] will be equal and opposite;
equilibrium point identified / two curves level off at same time;
Above 775 K: \({\text{rate}} = k{\text{[N}}{{\text{O}}_2}{\text{][CO]}}\);
Below 775 K: \({\text{rate}} = k{{\text{[N}}{{\text{O}}_2}{\text{]}}^2}\);
zero order reaction;
all concentrations are \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\);
\({\text{slope}} = \frac{{9.2 - 8.4}}{{(3.53 - 3.65) \times {{10}^{ - 3}}}} = - 6.67 \times {10^3}\);
\(({E_{\text{a}}} = 6.67 \times {10^3} \times 8.31)\)
\({\text{55.4 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept in range 55.0 – 56.0
Award [1] if 55454 (J) stated
Award [2] for the correct final answer
\(({K_{\text{c}}}) = \frac{{{\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}}}{{{\text{[C}}{{\text{l}}_2}{\text{][S}}{{\text{O}}_2}{\text{]}}}}\);
Ignore state symbols.
Square brackets [ ] required for the equilibrium expression.
\({\text{7.84}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol of S}}{{\text{O}}_2}\) and \({\text{7.84}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol of C}}{{\text{l}}_2}\);
\({\text{7.84}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ of S}}{{\text{O}}_2}\), \({\text{7.84}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ of C}}{{\text{l}}_2}\) and
\({\text{7.65}} \times {\text{1}}{{\text{0}}^{ - 4}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ of S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}\);
12.5;
Award [1] for 10.34
Award [3] for the correct final answer
value of \({K_{\text{c}}}\) increases;
\({\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}\) increases;
decrease in temperature favours (forward) reaction which is exothermic;
Do not allow ECF.
no effect on the value of \({K_{\text{c}}}\) / depends only on temperature;
\({\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}\) decreases;
increase in volume favours the reverse reaction which has more gaseous moles;
Do not allow ECF.
no effect;
catalyst increases the rate of forward and reverse reactions (equally) / catalyst decreases activation energies (equally);
Examiners report
In part (a) the rate expression was correctly stated although some confused this with an equilibrium constant expression.
Only the better candidates realized that the rate of reaction will decrease by a factor of four and there will be no effect on the rate constant.
Although most candidates were able to correctly sketch the concentration versus time graph many forgot to label the axes or include units.
Part (b) was well answered and candidates demonstrated a good understanding of rate expressions based on reaction mechanism.
The better candidates were able to figure out that the rate of a chemical reaction is equal to the rate constant when all concentrations are \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) or for a zero order reaction.
Most candidates had difficulty in calculating activation energy from the graph in part (d) and some gave the answer in \({\text{J}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) instead of \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) which showed that they missed this instruction in the question.
In part (e), the equilibrium constant expression was correctly stated by the majority but calculating the value of\({K_{\text{c}}}\) proved to be difficult.
A large number of candidates obtained the incorrect answer of 10.34 as a result of using the initial concentrations of the reactants instead of equilibrium concentrations.
The application of Le Chatelier’s principle was handled well by the majority with minor omissions such as not using the term gaseous particles in part (iv).
Some candidates stated that the addition of a catalyst does not affect the value of \({K_{\text{c}}}\) or the position of equilibrium, which did not answer the question and scored no marks because they had not commented on the concentration of \({\text{SOC}}{{\text{l}}_{\text{2}}}\). Some candidates correctly stated that a catalyst increases the rate of forward and reverse reactions equally.
Hydrogen gas reacts with iodine gas to form hydrogen iodide gas. A \({\text{2.00 d}}{{\text{m}}^{\text{3}}}\) flask was filled with \(1.50 \times {10^{ - 2}}{\text{ mol}}\) of hydrogen and \(1.50 \times {10^{ - 2}}{\text{ mol}}\) of iodine at a temperature, \(T\). The equilibrium constant, \({K_{\text{c}}}\), has a value of 53.0 at this temperature.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the formation of HI(g).
Determine the equilibrium concentrations, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), of hydrogen, iodine and hydrogen iodide.
Markscheme
\(({K_{\text{c}}} = )\frac{{{{{\text{[HI]}}}^2}}}{{{\text{[}}{{\text{H}}_2}{\text{][}}{{\text{I}}_2}{\text{]}}}}/\frac{{{\text{[HI]}}}}{{{{{\text{[}}{{\text{H}}_2}{\text{]}}}^{\frac{1}{2}}}{{{\text{[}}{{\text{I}}_2}{\text{]}}}^{\frac{1}{2}}}}}\);
Do not award mark if brackets are omitted or incorrect.
\(\begin{array}{*{20}{l}} {{{\text{H}}_{\text{2}}}}&{ + {{\text{I}}_{\text{2}}}}&{ \to {\text{2HI}}} \\ {(7.50 \times {{10}^{ - 3}} - x)}&{(7.50 \times {{10}^{ - 3}} - x)}&{{\text{2}}x/} \\ {(1.50 \times {{10}^{ - 2}} - x)}&{(1.50 \times {{10}^{ - 2}} - x)}&{{\text{2}}x/} \\ {{{{\text{[}}{{\text{H}}_{\text{2}}}{\text{]}}}_{{\text{initial}}}} - x}&{{{{\text{[}}{{\text{I}}_{\text{2}}}{\text{]}}}_{{\text{initial}}}} - x}&{{\text{2}}x{\text{;}}} \end{array}\)
Accept [H2]\(_{initial}\) = [I2]\(_{initial}\) = 7.50 \( \times \) 10–3 (mol\(\,\)dm–3) for M1.
\(53 = \frac{{{{(2x)}^2}}}{{{{(7.50 \times {{10}^{ - 3}} - x)}^2}}}/\sqrt {53} = \frac{{(2x)}}{{(7.50 \times {{10}^{ - 3}} - x)}}\);
Accept 53 \( = \frac{{{{(2x)}^2}}}{{{{(1.50 \times 1{0^{ - 2}} - x)}^2}}}/\sqrt {53} = \frac{{(2x)}}{{(1.50 \times 1{0^{ - 2}} - x)}}\)
\([{{\text{H}}_{\text{2}}}] = 1.62 \times {10^{ - 3}}{\text{ }}({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}})\) and \([{{\text{I}}_2}] = 1.62 \times {10^{ - 3}}{\text{ }}({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}})\);
\({\text{[HI]}} = 1.18 \times {10^{ - 2}}\);
Award [4] for correct final answer for values given in M3 and M4.
Award [2 max] for [H2] = [I2] = 7.50 \( \times \) 10–3 (mol\(\,\)dm–3) and
[HI] = 5.46 \( \times \) 10\(^{ - 2}\) mol\(\,\)dm–3.
OR
if \({K_{\text{c}}} = \frac{{{\text{[HI]}}}}{{{{{\text{[}}{{\text{H}}_{\text{2}}}{\text{]}}}^{\frac{1}{2}}}{{{\text{[}}{{\text{I}}_{\text{2}}}{\text{]}}}^{\frac{1}{2}}}}}\) is given in (i).
\(\begin{array}{*{20}{l}} {\frac{1}{2}{{\text{H}}_{\text{2}}}}&{ + \frac{1}{2}{{\text{I}}_{\text{2}}}}&{ \to {\text{HI}}} \\ {(7.50 \times {{10}^{ - 3}} - x)}&{(7.50 \times {{10}^{ - 3}} - x)}&{{\text{2}}x/} \\ {(1.50 \times {{10}^{ - 2}} - x)}&{(1.50 \times {{10}^{ - 2}} - x)}&{{\text{2}}x/} \\ {{{{\text{[}}{{\text{H}}_{\text{2}}}{\text{]}}}_{{\text{initial}}}} - x}&{{{{\text{[}}{{\text{I}}_{\text{2}}}{\text{]}}}_{{\text{initial}}}} - x}&{{\text{2}}x{\text{;}}} \end{array}\)
Accept [H2]\(_{initial}\) = [I2]\(_{initial}\) = 7.50 \( \times \) 10–3 (mol\(\,\)dm–3) for M1.
\(53 = \frac{{(2{\text{x}})}}{{(7.50 \times {{10}^{ - 3}} - x)}}\);
Accept 53 \( = \frac{{(2x)}}{{(1.50 \times 1{0^{ - 2}}- x)}}\).
\([{{\text{H}}_2}] = 2.73 \times {10^{ - 4}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \([{{\text{I}}_2}] = 2.73 \times {10^{ - 4}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\);
\([{\text{HI}}] = 1.45 \times {10^{ - 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\);
Award [4] for correct final answer for values given in M3 and M4.
Award [2 max] for [H2] = [I2] = 7.50 \( \times \) 10–3 (mol\(\,\)dm–3) and
[HI] = 5.46 \( \times \) 10\(^{ - 2}\) mol\(\,\)dm–3.
Examiners report
Most candidates scored poor marks in this question because they made mistakes in writing the correct equilibrium concentrations for H2, I2 and HI or calculating the value of ‘x’. It was not necessary to solve he quadratic equation to calculate the equilibrium concentrations. Most candidates knew that dimethylamine could form H-bonding with water, but only very few scored the mark for explanation. Reference to electronegativity or the lone pair of electrons on nitrogen was needed for both marks. Few candidates were able to name the amine correctly.
Most candidates scored poor marks in this question because they made mistakes in writing the correct equilibrium concentrations for H2, I2 and HI or calculating the value of ‘x’. It was not necessary to solve he quadratic equation to calculate the equilibrium concentrations. Most candidates knew that dimethylamine could form H-bonding with water, but only very few scored the mark for explanation. Reference to electronegativity or the lone pair of electrons on nitrogen was needed for both marks. Few candidates were able to name the amine correctly.
The vapour pressure of water changes with temperature according to the graph below.

A liquid boils when its vapour pressure equals atmospheric pressure. Determine the boiling point of water on a mountaintop on a day when the atmospheric pressure is 60.0 kPa.
Sketch another curve on the axes above to show how the vapour pressure of a liquid that has weaker intermolecular forces than water, such as bromine, changes with temperature.
(i) A sample of liquid bromine was left in a closed conical (Erlenmeyer) flask at 298 K and allowed to reach a state of equilibrium. State an observation that indicates that equilibrium was reached.
(ii) The temperature of the closed flask was increased and the system was allowed to reach a new equilibrium. Compare the equilibrium formed at the new temperature with the equilibrium at the original temperature on the molecular level.
Markscheme
87 (°C);
Accept boiling points in the range 86–88 °C.
similar shape above current curve / steeper than current curve;
Do not penalize if curves meet at 0 °C.
(i) (intensity of) colour of vapour is constant;
Accept volume/level of liquid is constant.
Allow pressure is constant.
(ii) more (molecules in the) vapour / fewer molecules in the liquid at new equilibrium / OWTTE;
molecules have more energy/move faster/collide more frequently at the new temperature / OWTTE;
rates of evaporation and condensation are higher at the new temperature;
in both flasks the rates of evaporation and condensation are equal;
Accept converse points for the flask at lower temperature for M1, M2 and M3.
Examiners report
A very well answered question.
Most candidates presented a curve that was steeper than the water vapour curve gaining the mark. However, most of the candidates started from the same vapour pressure as water at 0 °C which was not penalized. Very few candidates drew an accurate curve.
(i) This question was not well answered. Only a few candidates were able to give an appropriate observation. Many candidates could state the characteristics of a system in equilibrium but did not apply their knowledge to state an observation.
(ii) Only a few candidates gave adequate explanations gaining two marks. Many obtained one mark for saying that more molecules will be in the gaseous state. The reference to “molecular level” often went unnoticed.
In acidic solution, ions containing titanium can react according to the half-equation below.
\({\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\) \({E^\Theta } = - 0.06{\text{ V}}\)
In the diagram below, A and B are inert electrodes and, in the aqueous solutions, all ions have a concentration of \({\text{1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).

Sodium, silicon and sulfur are elements in period 3 of the periodic table that all form oxides.
Although carbon and silicon both belong to group 4 of the periodic table, carbon dioxide and silicon dioxide are different in many ways.
Define the term standard electrode potential, \({E^\Theta }\).
State the initial and final oxidation numbers of titanium and hence deduce whether it is oxidized or reduced in this change.

Considering the above equilibrium, predict, giving a reason, how adding more acid would affect the strength of the \({\text{Ti}}{{\text{O}}^{2 + }}\) ion as an oxidizing agent.
In the two experiments below, predict whether a reaction would occur and deduce an equation for any reaction that takes place. Refer to Table 14 of the Data Booklet if necessary.
KI(aq) is added to a solution containing \({\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}}\) ions:
Zn (s) is added to a solution containing \({\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}}\) and \({{\text{H}}^ + }{\text{(aq)}}\) ions:
Using Table 14 of the Data Booklet, state the balanced half-equation for the reaction that occurs at electrode A and whether it involves oxidation or reduction.
Calculate the cell potential in V.
On the diagram above label with an arrow
• the direction of electron flow in the wire
• the direction in which the positive ions flow in the salt bridge.
Compare the properties of the three oxides by completing the table below.

Sulfur dioxide is a significant contributor to acid deposition. Identify a major, man-made source of this pollutant.
As well as the oxide above, sodium forms a peroxide that contains the peroxide ion, \({\text{O}}_2^{2 - }\). Draw the Lewis (electron dot) structure of the peroxide ion.
Describe the differences in the hybridization of these group 4 elements and the precise nature of the bonds that they form with the oxygen atoms.
Xenon, although a noble gas, forms an oxide, \({\text{Xe}}{{\text{O}}_{\text{2}}}\), that has a structure related to that of \({\text{Si}}{{\text{O}}_{\text{2}}}\). Compare the geometry around the silicon atoms in \({\text{Si}}{{\text{O}}_{\text{2}}}\) with the geometry around the xenon atoms in \({\text{Xe}}{{\text{O}}_{\text{2}}}\), using the valence shell electron pair repulsion (VSEPR) theory.
Markscheme
potential of the half-cell / reduction half-reaction under standard conditions measured relative to standard hydrogen electrode/SHE;
Allow instead of standard conditions, solute concentration of 1 mol dm–3 or 1 bar/1 atm (pressure) for gases.

+ sign must be present. Do not award mark for incorrect notation 4, 4+, 3, 3+ etc.
Do not award M2 if inconsistent with M1.
increases / makes it stronger;
(more \({{\text{H}}^ + }\) would) drive/shift equilibrium to the right/towards products (accepting more electrons);
KI(aq) is added to a solution containing Ti3+(aq) ions:
no reaction;
Zn(s) is added to a solution containing TiO2+(aq) and H+(aq) ions:
\({\text{Zn(s)}} + {\text{2Ti}}{{\text{O}}^{2 + }}{\text{(aq)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{Z}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{2T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
correct reactants and products;
balanced equation;
Ignore state symbols.
\({\text{F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {{\text{e}}^ - } \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\);
Ignore state symbols.
Accept equilibrium arrow.
reduction;
Do not apply ECF.
\(( + 0.77 - ( - 0.06)) = ( + )0.83{\text{ (V)}}\);
Do not accept –0.83 V.
wire and salt bridge both have arrows from B to A;
Accept arrows above or below each provided it is obvious which they refer to.
Apply ECF from part (i).

For any parts (properties) where mark not awarded, award [1] for every three correct responses.
(combustion of) coal / diesel;
Accept “burning of fossil fuels”, “industrial processes” or “combustion/car engines”.
Do not accept “Contact process”.

e-pairs correct;
charges in correct positions;
Accept lines, or pairs of dots or crosses, for electron pairs.
Accept  .
.
C is sp hybridized and Si is \({\text{s}}{{\text{p}}^{\text{3}}}\) hybridized;
C–O bond in \({\text{C}}{{\text{O}}_{\text{2}}}\) has one \(\sigma \)-bond and one \(\pi \)-bond;
Si–O bond in \({\text{Si}}{{\text{O}}_{\text{2}}}\) has one \(\sigma \)-bond only;
Award [1 max] for last two marking points for “C–O double bond and Si–O single bond”.
silicon-oxygen bonds will have a tetrahedral distribution;
xenon-oxygen bonds will have a square planar distribution;
xenon dioxide has two non-bonding/lone pairs of electrons;
Award any of the above marks if clearly indicated in suitable diagrams.
Examiners report
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
An equilibrium exists between nitrosyl chloride, NOCl, nitrogen oxide, NO, and chlorine, \({\text{C}}{{\text{l}}_{\text{2}}}\).
\[{\text{2NOCl(g)}} \rightleftharpoons {\text{2NO(g)}} + {\text{C}}{{\text{l}}_2}{\text{(g)}}\]
\({\text{20.0 c}}{{\text{m}}^{\text{3}}}\) of hexane, \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{14}}}}\), and \({\text{20.0 c}}{{\text{m}}^{\text{3}}}\) of pentan-1-ol, \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\), were placed separately into two closed containers at 298 K and allowed to reach equilibrium.
Ammonia is a weak base.
(i) Deduce the equilibrium constant expression for this reaction.
(ii) Explain the effect on the position of equilibrium and the value of \({K_{\text{c}}}\) when pressure is decreased and temperature is kept constant.
(iii) 2.00 mol of NOCl was placed in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) container and allowed to reach equilibrium at 298 K. At equilibrium, 0.200 mol of NO was present. Determine the equilibrium concentrations of NOCl and \({\text{C}}{{\text{l}}_{\text{2}}}\), and hence calculate the value of \({K_{\text{c}}}\) at this temperature.
(iv) The value of \({K_{\text{c}}}\) is \(1.60 \times {10^{ - 5}}\) at 318 K. State and explain whether the forward reaction is exothermic or endothermic.
(i) Compare the two liquids in terms of their boiling points, enthalpies of vaporization and vapour pressures.
(ii) Explain your answer given for part (b)(i).
Calculate the pH of a \({\text{1.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ammonia at 298 K to two decimal places, using Table 15 of the Data Booklet.
A buffer solution is made using \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid, HCl (aq), and \({\text{20.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution, \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(aq)}}\).
Describe the meaning of the term buffer solution.
Determine the pH of the buffer solution at 298 K.
A \({\text{1.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ammonia is added to \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution in a titration experiment.
Calculate the total volume of the solution at the equivalence point.
Calculate the pH of the solution at the equivalence point, using Table 15 of the Data Booklet.
Identify a suitable indicator for this titration, using Table 16 of the Data Booklet.
Markscheme
(i) \(({K_{\text{c}}} = )\frac{{{\text{[C}}{{\text{l}}_2}{\text{(g)][NO(g)}}{{\text{]}}^2}}}{{{{{\text{[NOCl(g)]}}}^2}}}\);
Ignore state symbols.
(ii) equilibrium shifts to right as there are more moles (of gas) on product side;
no change to \({K_{\text{c}}}\) as it is a constant at fixed temperature / OWTTE;
(iii) \({\text{[NOCl(g)]}} = 1.80{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[C}}{{\text{l}}_2}{\text{(g)]}} = 0.100{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({K_{\text{c}}} = \left( {\frac{{0.100 \times {{(0.200)}^2}}}{{{{(1.80)}^2}}}} \right)1.23 \times {10^{ - 3}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
Award [3] for correct final answer.
(iv) exothermic as \({K_{\text{c}}}\) is lower at higher temperature;
(i) hexane has lower boiling point and enthalpy of vaporization than pentan-1-ol / OWTTE;
hexane has higher vapour pressure than pentan-1-ol / OWTTE;
(ii) hexane is non-polar / has only van der Waals’/London/dispersion forces / has weaker intermolecular forces than pentan-1-ol;
pentan-1-ol has hydrogen bonding between molecules;
\({\text{[O}}{{\text{H}}^ - }{\text{]}} = \sqrt {1.50 \times 1.78 \times {{10}^{ - 5}}} = 5.17 \times {10^{ - 3}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{pH}} = (14 - {\text{pOH}} = 14 - 2.29 = ){\text{ }}11.71\);
Award [2] for correct final answer.
Accept correct answer with more than 2 decimal places.
solution which resists change in pH / changes pH slightly / OWTTE;
when small amounts of acid or base are added;
\({\text{[N}}{{\text{H}}_3}{\text{] = }}\left( {\frac{{(1.50 \times 0.0200) - (0.500 \times 0.0250)}}{{0.0450}} = } \right){\text{ }}0.389{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[NH}}_4^ + {\text{]}} = \left( {\frac{{(0.500 \times 0.0250)}}{{0.0450}} = } \right){\text{ }}0.278{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[O}}{{\text{H}}^ - }{\text{]}} = \left( {\frac{{{K_b}{\text{[N}}{{\text{H}}_3}{\text{]}}}}{{{\text{[NH}}_4^ + {\text{]}}}} = } \right){\text{ }}\frac{{1.78 \times {{10}^{ - 5}} \times 0.389}}{{0.278}} = 2.49 \times {10^{ - 5}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{pH}} = (14.0 - {\text{pOH}} = 14.0 - 4.60 = ){\text{ }}9.40\);
OR
\({\text{pOH}} = {\text{p}}{K_b} + \log \frac{{[{\text{NH}}_4^ + ]}}{{{\text{[N}}{{\text{H}}_3}]}}{\text{ = p}}{K_{\text{b}}} + \log \frac{{(12.5/1000)}}{{(17.5/1000)}}\);
\({\text{pOH}} = 4.75 + \log \left( {\frac{{12.5}}{{17.5}}} \right) = 4.75 - 0.146 = 4.604\);
\({\text{pH}} = 14.0 - 4.604 = 9.40\);
Award [4] for the correct final answer.
\(\left( {{\text{V(N}}{{\text{H}}_{\text{3}}}{\text{)}} = \frac{{25.0 \times 0.500}}{{1.50}} = 8.33{\text{ c}}{{\text{m}}^3}} \right)\)
\({\text{V}} = {\text{V(N}}{{\text{H}}_3}{\text{)}} + {\text{V(HCl)}} = 8.33 + 25.0 = 33.3{\text{ c}}{{\text{m}}^3}/0.0333{\text{ d}}{{\text{m}}^3}\);
(\({\text{NH}}_{\text{4}}^ + \) ions are present at equivalence point \({\text{N}}{{\text{H}}_3} + {\text{HCl}} \to {\text{NH}}_4^ + + {\text{C}}{{\text{l}}^ - }\) at equivalence \({\text{n}}({\text{NH}}_4^ + {\text{ produced}}) = {\text{n}}({\text{N}}{{\text{H}}_3}{\text{ added}}) = {\text{n(HCl)}}\))
\([{\text{NH}}_4^ + ] = \frac{{0.500 \times 0.0250}}{{0.0333}} = 0.375{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}})\);
\({\text{(NH}}_4^ + {\text{(aq)}} \rightleftharpoons {\text{N}}{{\text{H}}_3}{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}}/{\text{NH}}_4^ + {\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{N}}{{\text{H}}_3}{\text{(aq)}} + {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}}\)
\({\text{p}}{K_{\text{a}}}{\text{(NH}}_4^ + ) = 14 - {\text{p}}{K_{\text{b}}}{\text{(N}}{{\text{H}}_3}) = 14.00 - 4.75 = 9.25)\)
\({K_{\text{a}}} = \frac{{{\text{[N}}{{\text{H}}_3}{\text{(aq)][}}{{\text{H}}^ + }{\text{(aq)]}}}}{{{\text{[NH}}_4^ + {\text{(aq)]}}}} = 5.62 \times {10^{ - 10}}\);
\({\text{[}}{{\text{H}}^ + }{\text{(aq)]}} = \sqrt {5.62 \times {{10}^{ - 10}} \times 0.375} = 1.45 \times {10^{ - 5}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{pH}} = 4.84\);
Award [4] for the correct final answer.
bromocresol green / methyl red;
ECF for answer in 7(c)(v) if pH given is below 7.
Examiners report
The construction and use of equilibrium expressions for \({K_{\text{c}}}\) showed good understanding. The prediction of the effect of increasing pressure on the position of equilibria by applying Le Chatelier’s principle was good, but the fact that \({K_{\text{c}}}\) remains constant at fixed temperatures was less well known.
pH calculations in c(i), c(ii) and c(v) tended to be very good or completely incorrect.
The construction and use of equilibrium expressions for \({K_{\text{c}}}\) showed good understanding. The prediction of the effect of increasing pressure on the position of equilibria by applying Le Chatelier’s principle was good, but the fact that \({K_{\text{c}}}\) remains constant at fixed temperatures was less well known.
pH calculations in c(i), c(ii) and c(v) tended to be very good or completely incorrect.
The construction and use of equilibrium expressions for \({K_{\text{c}}}\) showed good understanding. The prediction of the effect of increasing pressure on the position of equilibria by applying Le Chatelier’s principle was good, but the fact that \({K_{\text{c}}}\) remains constant at fixed temperatures was less well known.
pH calculations in c(i), c(ii) and c(v) tended to be very good or completely incorrect.
The construction and use of equilibrium expressions for \({K_{\text{c}}}\) showed good understanding. The prediction of the effect of increasing pressure on the position of equilibria by applying Le Chatelier’s principle was good, but the fact that \({K_{\text{c}}}\) remains constant at fixed temperatures was less well known.
pH calculations in c(i), c(ii) and c(v) tended to be very good or completely incorrect.
The construction and use of equilibrium expressions for \({K_{\text{c}}}\) showed good understanding. The prediction of the effect of increasing pressure on the position of equilibria by applying Le Chatelier’s principle was good, but the fact that \({K_{\text{c}}}\) remains constant at fixed temperatures was less well known.
pH calculations in c(i), c(ii) and c(v) tended to be very good or completely incorrect.
The construction and use of equilibrium expressions for \({K_{\text{c}}}\) showed good understanding. The prediction of the effect of increasing pressure on the position of equilibria by applying Le Chatelier’s principle was good, but the fact that \({K_{\text{c}}}\) remains constant at fixed temperatures was less well known.
pH calculations in c(i), c(ii) and c(v) tended to be very good or completely incorrect.
The construction and use of equilibrium expressions for \({K_{\text{c}}}\) showed good understanding. The prediction of the effect of increasing pressure on the position of equilibria by applying Le Chatelier’s principle was good, but the fact that \({K_{\text{c}}}\) remains constant at fixed temperatures was less well known.
pH calculations in c(i), c(ii) and c(v) tended to be very good or completely incorrect.
The construction and use of equilibrium expressions for \({K_{\text{c}}}\) showed good understanding. The prediction of the effect of increasing pressure on the position of equilibria by applying Le Chatelier’s principle was good, but the fact that \({K_{\text{c}}}\) remains constant at fixed temperatures was less well known.
pH calculations in c(i), c(ii) and c(v) tended to be very good or completely incorrect.
The rate of reaction is an important factor in industrial processes such as the Contact process to make sulfur trioxide, \({\text{S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\).
Define the term rate of reaction.
Describe the collision theory.
The Contact process involves this homogeneous equilibrium:
\[{\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\,\,\,\,\,\Delta H = - 198{\text{ kJ}}\]
State and explain how increasing the pressure of the reaction mixture affects the yield of \({\text{S}}{{\text{O}}_{\text{3}}}\).
The Contact process involves this homogeneous equilibrium:
\[{\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\,\,\,\,\,\Delta H = - 198{\text{ kJ}}\]
2.00 mol of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{(g)}}\) are mixed with 3.00 mol of \({{\text{O}}_{\text{2}}}{\text{(g)}}\) in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) container until equilibrium is reached. At equilibrium there are 0.80 mol of \({\text{S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\).
Determine the equilibrium constant (\({K_{\text{c}}}\)) assuming all gases are at the same temperature and pressure.
The Contact process involves this homogeneous equilibrium:
\[{\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\,\,\,\,\,\Delta H = - 198{\text{ kJ}}\]
State the effect of increasing temperature on the value of \({K_{\text{c}}}\) for this reaction.
Outline the economic importance of using a catalyst in the Contact process.
Markscheme
change in concentration of reactant/product with time / rate of change of concentration;
Accept “increase” instead of “change” for product and “decrease” instead of “change” for reactant.
Accept “mass/amount/volume” instead of “concentration”.
Do not accept substance.
collision frequency;
two particles must collide;
particles must have sufficient energy to overcome the activation energy/\(E \geqslant {E_a}\);
Concept of activation energy must be mentioned.
appropriate collision geometry/orientation;
increases yield;
(equilibrium shifts to the right/products as) more gaseous moles in reactants/on left / fewer gaseous moles in products/on right;
\({\text{Eqm[}}{{\text{O}}_2}{\text{]}} = {\text{2.6 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{Eqm[S}}{{\text{O}}_2}{\text{]}} = {\text{1.2 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({K_{\text{c}}} = \frac{{{{{\text{[S}}{{\text{O}}_3}]}^2}}}{{{{{\text{[S}}{{\text{O}}_2}{\text{]}}}^2}{\text{[}}{{\text{O}}_2}{\text{]}}}}\);
\({K_{\text{c}}} = 0.17\);
Award [4] for correct final answer.
Ignore units.
\({\text{(}}{K_{\text{c}}}{\text{)}}\) decreases;
catalyst increases rate of reaction / equilibrium reached faster / increases yield of product per unit time;
reduces costs / reduces energy needed;
Do not accept just “increases the yield”.
Examiners report
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
Consider the following equilibrium.
\[\begin{array}{*{20}{l}} {{\text{2S}}{{\text{O}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_3}{\text{(g)}}}&{\Delta {H^\Theta } = - 198{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
State and explain the effect of increasing the pressure on the yield of sulfur trioxide.
State the effects of a catalyst on the forward and reverse reactions, on the position of equilibrium and on the value of \({K_{\text{c}}}\).
When a mixture of 0.100 mol NO, 0.051 mol \({{\text{H}}_{\text{2}}}\) and 0.100 mol \({{\text{H}}_{\text{2}}}{\text{O}}\) were placed in a \({\text{1.0 d}}{{\text{m}}^{\text{3}}}\) flask at 300 K, the following equilibrium was established.
\(2{\text{NO(g)}} + 2{{\text{H}}_2}{\text{(g)}} \rightleftharpoons {{\text{N}}_2}{\text{(g)}} + 2{{\text{H}}_2}{\text{O(g)}}\)
At equilibrium, the concentration of NO was found to be \({\text{0.062 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the equilibrium constant, \({K_{\text{c}}}\), of the reaction at this temperature.
Outline two differences between an electrolytic cell and a voltaic cell.
Electroplating is an important application of electrolysis. State the composition of the electrodes and the electrolyte used in the silver electroplating process.
Markscheme
yield (of \({\text{S}}{{\text{O}}_{\text{3}}}\)) increases / equilibrium moves to right / more \({\text{S}}{{\text{O}}_{\text{3}}}\) formed;
3 gaseous molecules \( \to \) 2 gaseous molecules / decrease in volume of gaseous molecules / fewer gaseous molecules on right hand side;
Do not allow ECF.
rates of both forward and reverse reactions increase equally;
no effect on position of equilibrium;
no effect on value of [3] \({K_{\text{c}}}\);
\({\text{2NO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \rightleftharpoons {{\text{N}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\)

\({\text{[}}{{\text{H}}_{\text{2}}}{\text{] at equilibrium}} = 0.013{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[}}{{\text{N}}_{\text{2}}}{\text{] at equilibrium}} = 0.019{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[}}{{\text{H}}_{\text{2}}}{\text{O] at equilibrium}} = 0.138{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({K_{\text{c}}} = {\text{[}}{{\text{N}}_2}{\text{][}}{{\text{H}}_2}{\text{O}}{{\text{]}}^2}{\text{/[NO}}{{\text{]}}^2}{{\text{[}}{{\text{H}}_2}{\text{]}}^2} = {\text{(0.019)(0.138}}{{\text{)}}^2}{\text{/(0.062}}{{\text{)}}^2}{{\text{(0.013)}}^2} = 5.6 \times {10^2}\);
Award [4] for final correct answer.
Accept any value also in range 557–560.
Do not penalize significant figures.
electrolytic cell converts electrical energy to chemical energy and voltaic cell converts chemical energy to electrical energy / electrolytic cell uses electricity to carry out a (redox) chemical reaction and voltaic cell uses a (redox) chemical reaction to produce electricity / electrolytic cell requires a power supply and voltaic cell does not;
electrolytic cell involves a non-spontaneous (redox) reaction and voltaic cell involves a spontaneous (redox) reaction;
in an electrolytic cell, cathode is negative and anode is positive and vice-versa for a voltaic cell / electrolytic cell, anode is positive and voltaic cell, anode is negative / electrolytic cell, cathode is negative and voltaic cell, cathode is positive;
voltaic cell has two separate solutions and electrolytic cell has one solution / voltaic cell has salt bridge and electrolytic cell has no salt bridge;
electrolytic cell, oxidation occurs at the positive electrode/anode and voltaic cell, oxidation occurs at the negative electrode/anode and vice-versa;
Cathode/negative electrode:
object to be plated;
Allow a specific example here e.g. spoon.
Accept inert metal/graphite.
Do not accept silver halides or their formulae.
Anode/positive electrode:
Silver/Ag;
Electrolyte:
\({{\text{[Ag(CN}}{{\text{)}}_{\text{2}}}{\text{]}}^ - }\);
Allow silver nitrate/AgNO3 / silver cyanide/any other suitable silver salt/solution.
Do not accept AgCl.
Examiners report
In (ii) an overwhelming number of candidates were able to score the first mark but did not refer to the gaseous state and hence lost the second mark.
Part (iv) was another question where candidates easily scored the second and third mark. Although this has been asked a number of times in recent sessions, some candidates still do not state that the rates of both the forward and reverse reactions increase equally.
(b) was considered a very challenging question for candidates, and usually only the better candidates scored all four marks.
In (c) (i) most candidates scored two marks.
Electroplating was a topic only partially understood by candidates, and so only a few candidates obtained all three marks in (v). Often the nature of the electrode was mixed up or in many cases incorrect electrolytes were given.
Define the terms acid and base according to the Brønsted-Lowry theory. Distinguish between a weak base and a strong base. State one example of a weak base.
Weak acids in the environment may cause damage. Identify a weak acid in the environment and outline one of its effects.
The graph below indicates the pH change during the titration of \({\text{20.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH(aq)}}\) with \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ KOH(aq)}}\). From the graph, identify the volume of KOH(aq) and the pH at the equivalence point.

Explain how the graph could be used to determine the \({\text{p}}{K_{\text{a}}}\) of ethanoic acid and determine the \({\text{p}}{K_{\text{a}}}\) value for these data.
Sketch a graph, similar to the graph on the previous page, to indicate the change in pH during a titration of \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ HN}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\) with \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) KOH(aq). On your graph, clearly indicate the starting pH value, the equivalence point, the pH at the equivalence point and the final pH reached.
Describe how an indicator works.
Using Table 16 of the Data Booklet, identify the most appropriate indicator for the titration of ethanoic acid with potassium hydroxide. Explain your choice.
Determine the pH of the solution resulting when \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ HCl(aq)}}\) is mixed with \({\text{200 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ NaOH(aq)}}\).
Markscheme
Acid: proton/\({{\text{H}}^ + }\) donor and Base: proton/\({{\text{H}}^ + }\) acceptor;
Do not accept \(O{H^ - }\) for base.
Weak base: (base/electrolyte) partially dissociated/ionized (in solution/water) and Strong base: (base/electrolyte assumed to be almost) completely/100% dissociated/ionized (in solution/water) / OWTTE;
\({\text{N}}{{\text{H}}_{\text{3}}}/{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\);
Allow either name or formula or other suitable example.
sulfurous acid/\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{3}}}\);
corrodes marble/limestone buildings/statues / leaching in soils / harms/kills
plants;
OR
nitrous acid/\({\text{HN}}{{\text{O}}_{\text{2}}}\);
corrodes marble/limestone buildings/statues / leaching in soils / harms/kills
plants;
OR
carbonic acid/\({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\);
corrodes marble/limestone buildings/statues / acidification of lakes;
Do not allow oxides (e.g. CO2 etc.).
Do not accept just corrodes or damages.
Volume of KOH: 20 (\({\text{c}}{{\text{m}}^{\text{3}}}\));
Allow any value between 20 and 21 (cm3).
pH at the equivalence point: 8.0–10.0;
At half-equivalence point \({\text{[C}}{{\text{H}}_{\text{3}}}{\text{COOH]}} = {\text{[C}}{{\text{H}}_{\text{3}}}{\text{CO}}{{\text{O}}^ - }{\text{]}}\) so \({\text{pH}} = {\text{p}}{K_{\text{a}}}\);
\({\text{p}}{K_{\text{a}}} = 4.7\);
Accept in range 4.2 to 5.2.
M2 can only be scored if M1 correct (i.e. no marks for just Data Booklet value of 4.76).

Starting pH: 1;
Equivalence point: \({\text{pH}} = 7\) and 25 \({\text{c}}{{\text{m}}^{\text{3}}}\);
Final pH reached: 12–13;
correct curve shape;
Do not award M4 if turn in curve is seen at low volumes (suggesting weak acid–strong base titration).
Award [4] if values corresponding to M1, M2 and M3 are labelled on graph (e.g using X) and correct shape of curve shown.
HIn is a weak acid / weak base;

Award [2] for M2 alone.
in base equilibrium moves to right / in acid equilibrium moves to left;
phenolphthalein;
indicator colour change occurs in range of pH at the equivalence point / OWTTE;
M2 can be scored independently even if indicator is incorrect.
\(n{\text{(HCl)}} = (0.100 \times 0.50) = 0.050{\text{ (mol)}}\);
\(n{\text{(NaOH)}} = (0.200 \times 0.10) = 0.020{\text{ (mol)}}\);
\(n{{\text{(HCl)}}_{{\text{remaining}}}} = (0.050 - 0.020) = 0.030{\text{ (mol)}}\);
\({\text{[HCl]}} = \left( {\frac{{0.030}}{{0.30}}} \right) = 0.10{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{pH}} = 1.0\);
Award [2 max] for just pH = 1.0 without working.
Examiners report
This was a popular question and often was well answered by candidates. In (a) (i) most candidates knew the formal definitions of an acid and a base and most could distinguish between a weak base and a strong base. Ammonia was generally given as a suitable example of a weak base. Some of the weaker students gave sodium hydroxide incorrectly as an example of a weak base which was quite surprising at HL.
In (ii), common mistakes included nitric acid and this question proved to be problematic for candidates. There were a number of G2 comments expressing some concern at asking this style of question, though this is a clear Aim 8 type question that should be explored in the formal teaching programme.
(iii) was well done.
Candidates rarely got (iv) correct.
In (v) most candidates scored either two or three, but often an incorrect shape of the curve was given.
In (b), few could describe how an indicator works and the equilibrium sign was sometimes omitted.
In (ii), phenolphthalein was usually identified as an appropriate indicator.
In (d), candidates who were able to think logically about all this did well; others scattered figures across the page and became hopelessly muddled. Often an incorrect answer of \({\text{pH}} = 7.0\) was seen.
The periodic table shows the relationship between electron configuration and the properties of elements and is a valuable tool for making predictions in chemistry.
The ten elements in the first-row d-block have characteristic properties and many uses.
Define the term electronegativity.
(i) Outline two reasons why a sodium ion has a smaller radius than a sodium atom.
(ii) Explain why the ionic radius of \({{\text{P}}^{3 - }}\) is greater than the ionic radius of \({\text{S}}{{\text{i}}^{4 + }}\).
The graph below represents the successive ionization energies of sodium. The vertical axis plots log (ionization energy) instead of ionization energy to allow the data to be represented without using an unreasonably long vertical axis.

State the full electron configuration of sodium and explain how the successive ionization energy data for sodium are related to its electron configuration.
(i) Explain why the first ionization energy of aluminium is lower than the first ionization energy of magnesium.
(ii) Explain why the first ionization energy of sulfur is lower than the first ionization energy of phosphorus.
State and explain the type of reaction that takes place between \({\text{F}}{{\text{e}}^{3 + }}\) and \({{\text{H}}_{\text{2}}}{\text{O}}\) to form \({{\text{[Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) in terms of acid-base theories.
Explain why \({{\text{[Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) is coloured.
Outline the economic significance of the use of a catalyst in the Haber process which is an exothermic reaction.
Markscheme
power/strength/ability of an atom to attract electrons/shared electron pair / OWTTE;
in a (covalent) bond;
Accept the word “element” in place of “atom”.
Do not accept electron (singular).
(i) Na: 11 p, 11/ 2.8.1 \({{\text{e}}^ - }\) and \({\text{N}}{{\text{a}}^ + }\): 11 p, 10 / 2.8 \({{\text{e}}^ - }\) / same number of protons, less electrons / \({\text{N}}{{\text{a}}^ + }\) has 2 shells/energy levels, Na has 3 / OWTTE;
Na+: has greater net positive charge/same number of protons pulling smaller number of electrons;
(ii) Si4+: 10 \({{\text{e}}^ - }\) in 2 (filled) energy levels / electron arrangement 2.8 / OWTTE;
P3−: 18 \({{\text{e}}^ - }\) in 3 (filled) energy levels / electron arrangement 2.8.8, thus larger / OWTTE;
OR
\({\text{S}}{{\text{i}}^{4 + }}\): has 2 energy levels where as \({{\text{P}}^{3 - }}\) has 3/ \({{\text{P}}^{3 - }}\) has one more (filled) energy
level;
\({\text{S}}{{\text{i}}^{4 + }}\): 10 \({{\text{e}}^ - }\) in 2 energy levels where as \({{\text{P}}^{3 - }}\) has 18 \({{\text{e}}^ - }\), thus larger;
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{1}}}\);
Do not accept [Ne] 3s1.
first electron easy/easiest to remove / 1 electron in outermost/\({\text{n}} = 3\) energy level;
large increase between 1st and 2nd IE as electron now removed from \({\text{n}} = 2\) / next 8 electrons more difficult to remove / show (relatively) small increase as these electrons are in the same energy level/second energy level/\({\text{n}} = 2\);
large increase between 9th and 10th IE as electron now removed from n = 1 / 2
electrons very hard/most difficult to remove / innermost/lowest/closest to the nucleus/energy level/\({\text{n}} = 1\) / OWTTE;
electron 11 also comes from 1s, so shows a small increase;
(i) outer electron in Al is in 3p/p orbital/sub-shell/sub-level;
higher orbital/sub-shell / e– further from nucleus / shielded by 3s electrons;
(ii) in S, electron paired in 3p/p orbital/sub-shell/sub-level;
Accept extra stability associated with half filled p sub-shell (in P).
repulsion between paired electrons (and therefore easier to remove);
Lewis acid-base (reaction);
\({{\text{H}}_{\text{2}}}{\text{O}}\): e-pair donor, \({\text{F}}{{\text{e}}^{3 + }}\): \({{\text{e}}^ - }\) pair acceptor / \({{\text{H}}_{\text{2}}}{\text{O}}\) donates an electron pair to \({\text{F}}{{\text{e}}^{3 + }}\);
d sub-levels are split into two sets of orbitals (of different energies);
electron transitions between (d) orbitals of different energies / d-d transition(s);
transmitted (visible) light is complementary colour;
(exothermic reactions) low temperature/less energy increases ammonia yield;
(iron) catalyst used to increase rate of reaction / equilibrium reached faster / same yield but produced faster/in shorter/less time;
Examiners report
Generally, the definition of electronegativity was good, but some made the error of saying that it was the attraction of one electron only; others did not specify that it is the ability of an atom to attract a shared electron pair in a covalent bond.
Reasons why a sodium ion has a smaller radius than a sodium atom solicited incomplete answers. The answer requires the number of shells, electrons and protons of both the ion and the atom. Many candidates correctly said that \({\text{N}}{{\text{a}}^ + }\) had the same number of protons but one electron less so the pulling effect on the electrons was greater. Not many candidates gave the electronic structure or number of shells of the two ions, \({{\text{P}}^{3 - }}\) and \({\text{S}}{{\text{i}}^{4 + }}\), to explain their difference in ionic radius.
The graphical question on successive ionization energies of sodium was well answered by many. Typically, they explained how the successive ionization energies of sodium are related to its electron configuration from the data given. Most candidates realized that aluminium’s outer electron is in the 3p orbital so further from the nucleus and thus easier to ionize than magnesium. Similarly, sulfur has a paired electron in the 3p sub-shell and the repulsion between paired electrons is greater than in phosphorus which has a half filled p sub-shell.
Many candidates did not give sufficient answers to the part on transition elements. Some realised that it was a Lewis acid-base reaction where the electrons are donated by the water molecule to \({\text{F}}{{\text{e}}^{3 + }}\). Explanations given for the colour of complex ions continue to be muddled and the language used imprecise. Many wrote of “a split d orbital” rather than the d sub-level being split into two sets of orbitals (of different energies). The colour seen was often attributed to electrons emitting those wavelengths in transitions from higher energy to lower energy d orbitals rather than the transmitted visible light being the complementary colour of the one absorbed.
Few complete answers were given about economic significance of the use of a catalyst in the Haber process. A point that was missing often was that because the reaction is exothermic the forward reaction would be favoured (and the yield) if the temperature is lowered, but this would bring about a slower reaction so a catalyst is necessary to reach the equilibrium faster. However, there were misconceptions both in as far as catalysts and energetic is concerned. It was surprising to see the number of candidates who referred to activation energy but used the concept incorrectly. Few candidates established a connection with equilibrium.
Few complete answers were given about economic significance of the use of a catalyst in the Haber process. A point that was missing often was that because the reaction is exothermic the forward reaction would be favoured (and the yield) if the temperature is lowered, but this would bring about a slower reaction so a catalyst is necessary to reach the equilibrium faster. However, there were misconceptions both in as far as catalysts and energetic is concerned. It was surprising to see the number of candidates who referred to activation energy but used the concept incorrectly. Few candidates established a connection with equilibrium.
Few complete answers were given about economic significance of the use of a catalyst in the Haber process. A point that was missing often was that because the reaction is exothermic the forward reaction would be favoured (and the yield) if the temperature is lowered, but this would bring about a slower reaction so a catalyst is necessary to reach the equilibrium faster. However, there were misconceptions both in as far as catalysts and energetic is concerned. It was surprising to see the number of candidates who referred to activation energy but used the concept incorrectly. Few candidates established a connection with equilibrium.
Magnesium, a reactive metal found in many common minerals, is also an essential nutrient for both plants and animals.
Successive ionization energies of magnesium are given in the table below.

Magnesium metal is mainly used as a component in lightweight alloys, particularly in combination with aluminium and titanium.
Magnesium is usually produced by the electrolysis of molten magnesium chloride.
Define the term first ionization energy.
(i) Explain why the second ionization energy is greater than the first ionization energy.
(ii) Explain why the third ionization energy is much greater than the second ionization energy.
Although magnesium is usually found as \({\text{M}}{{\text{g}}^{2 + }}\) in its compounds, it is possible to use the Born-Haber cycle to investigate the possibility of \({\text{M}}{{\text{g}}^ + }\) being able to form stable compounds.
Use the ionization energy data from part (b), along with the other data provided below, to determine the enthalpy change of formation of MgCl(s). Assume that, because \({\text{M}}{{\text{g}}^ + }\) would be similar in size to \({\text{N}}{{\text{a}}^ + }\), MgCl would have a similar lattice enthalpy to NaCl.
Enthalpy of atomization of Mg \( + 146{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Bond enthalpy in \({\text{C}}{{\text{l}}_{\text{2}}}\) \( + 243{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Electron affinity of Cl \( + 349{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Lattice enthalpy of NaCl \( + 790{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Consider the lattice enthalpies of \({\text{Mg}}{{\text{F}}_{\text{2}}}\), \({\text{MgC}}{{\text{l}}_2}\) and \({\text{CaC}}{{\text{l}}_{\text{2}}}\). List these from the most endothermic to the least endothermic and explain your order.
\({\text{Most endothermic}} \to {\text{Least endothermic}}\)
Magnesium hydroxide, \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), is only sparingly soluble in water and the equilibrium below exists when excess solid is in contact with a saturated solution.
\[{\text{Mg(OH}}{{\text{)}}_2}{\text{(s)}} \rightleftharpoons {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}\]
Outline how the solubility of magnesium hydroxide will vary with pH.
(i) Describe the bonding present in magnesium metal.
(ii) Suggest why magnesium is harder than sodium.
(iii) Outline why alloys are generally less malleable than their component metals.
(i) Draw a labelled diagram of a suitable apparatus for the electrolysis.
(ii) State equations for the reactions that take place at the electrodes.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iii) When dilute aqueous magnesium chloride is used as the electrolyte, the reactions at both electrodes are different. State equations for the reactions that occur in aqueous solution.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iv) Outline why magnesium metal is not produced in the electrolysis of aqueous magnesium chloride.
Markscheme
minimum energy required to remove one electron / energy required to remove most loosely bound/outermost electron;
from gaseous/isolated atom;
Accept “gaseous state”.
More extensive definitions involving one mole may be given.
(i) electrons lost in same orbital/valence shell;
(second) electron/electron (being lost from \({\text{M}}{{\text{g}}^ + }\) is) closer to the nucleus;
(second) electron/electron (being lost from \({\text{M}}{{\text{g}}^ + }\)) not subject to e-e repulsion from others in same level;
Apply OWTTE for all marking points.
Do not accept “less electrons to share the charge” or answers employing this concept.
(ii) electron in lower energy level / more stable electron shell;
electron closer to nucleus;
less shielding by complete inner shells / increase in effective nuclear charge;
Apply OWTTE for all marking points.
\(\Delta {H_{{\text{at}}}}{\text{(Cl)}} = \frac{1}{2} \times 243{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Correct calculation of atomization enthalpy of Cl.
\(\Delta {H_{\text{f}}} = + 146 + \frac{1}{2}243 + 738 + ( - 349) + ( - 790)\);
Correct sign and magnitude of all terms.
\( = - {\text{134 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Final mark involves correct computation of equation the student has produced.
Award [2] for –12 (bond enthalpy of Cl not halved) or +134 (signs wrong).
Award [1] for +12 (bond enthalpy of Cl not halved and signs wrong).
\({\text{Mg}}{{\text{F}}_2}\) –\({\text{MgC}}{{\text{l}}_2}\) –\({\text{CaC}}{{\text{l}}_2}\);
\({{\text{F}}^ - }\) smaller (ionic radius) than \({\text{C}}{{\text{l}}^ - }\) / \({\text{C}}{{\text{l}}^ - }\) larger (ionic radius) than \({{\text{F}}^ - }\);
\({\text{M}}{{\text{g}}^{2 + }}\) smaller (ionic radius) than \({\text{C}}{{\text{a}}^{2 + }}\) / \({\text{C}}{{\text{a}}^{2 + }}\) larger (ionic radius) than \({\text{M}}{{\text{g}}^{2 + }}\);
Accept use of atomic radius rather than ionic radius.
more soluble at low pH / less soluble at high pH;
higher pH / \({\text{O}}{{\text{H}}^ - }\) will shift the equilibrium to the left / lower pH / \({{\text{H}}^ + }\) will (react with \({\text{O}}{{\text{H}}^ - }\) and) shift the equilibrium to the right;
(i) lattice/layers/framework of cations/magnesium ions/\({\text{M}}{{\text{g}}^{2 + }}\);
surrounded by delocalized electrons / in a sea/flux of delocalized electrons;
Accept “mobile” instead of “delocalized”.
(ii) Mg has more delocalized electrons (than Na);
Accept “Mg has more valence electrons than Na” / “Mg is Mg2+ but Na is only Na+”.
(iii) layers of ions/atoms/particles cannot slide over each other so easily (as different sized ions/atoms/particles) / OWTTE;
(i) 
Diagram:
two electrodes connected to a power pack/battery and immersed in an electrolyte;
Do not award mark if salt bridge included in diagram.
Labelling:
anode/positive electrode, cathode/negative electrode, molten magnesium chloride/MgCl2 (l)/electrolyte correctly labelled;
Check candidates know which end of a battery symbol is which charge.
(ii) Negative electrode (cathode): \({\text{M}}{{\text{g}}^{2 + }}{\text{(l)}} + {\text{2}}{{\text{e}}^ - } \to {\text{Mg (s)}}\);
Positive electrode (anode): \[{\text{2C}}{{\text{l}}^ - }{\text{(l)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }\];
Accept \(C{l^ - }(l) \to \frac{1}{2}C{l_2}(g) + {e^ - }\).
Ignore state symbols.
Allow e instead of e–.
If both correct equations are given for the wrong electrodes award [1 max].
(iii) Negative electrode (cathode):
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}/{\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}}\);
Accept \(4{H_2}O(l) + 4{e^ - } \to 2{H_2}(g) + 4O{H^ - }(aq) / 4{H^ + }(aq) + 4{e^ - } \to 2{H_2}(g)\) / \({H_2}O(l) + {e^ - } \to \frac{1}{2}{H_2}(g) + O{H^ - }(aq)/{H^ + }(aq) + {e^ - } \to \frac{1}{2}{H_2}(g)\).
Positive electrode (anode):
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{4}}{{\text{e}}^ - }/{\text{4O}}{{\text{H}}^ - }{\text{(aq)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{4}}{{\text{e}}^ - }\);
Accept \({H_2}O(l) \to \frac{1}{2}{O_2}(g) + 2{H^ + }(aq) + 2{e^ - } / 2O{H^ - }(aq) \to \frac{1}{2}{O_2}(g) + {H_2}O(l) + 2{e^ - }\).
State symbols not required.
Allow e instead of e–.
If both correct equations are given for the wrong electrodes award [1 max].
(iv) water/hydrogen ions more easily reduced/better oxidizing agents/have a more positive \({E^\Theta }\) (than magnesium ions);
Accept converse statements for magnesium ions.
Accept “magnesium is very reactive/high in reactivity series” / OWTTE.
Examiners report
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
Water is an important substance that is abundant on the Earth’s surface.
Buffer solutions resist small changes in pH. A phosphate buffer can be made by dissolving \({\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{4}}}\) and \({\text{N}}{{\text{a}}_{\text{2}}}{\text{HP}}{{\text{O}}_{\text{4}}}\) in water, in which \({\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{4}}}\) produces the acidic ion and \({\text{N}}{{\text{a}}_{\text{2}}}{\text{HP}}{{\text{O}}_{\text{4}}}\) produces the conjugate base ion.
A \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution is placed in a flask and titrated with a \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution.
(i) State the expression for the ionic product constant of water, \({K_{\text{w}}}\).
(ii) Explain why even a very acidic aqueous solution still has some \({\text{O}}{{\text{H}}^ - }\) ions present in it.
(iii) State and explain the effect of increasing temperature on the value of \({K_{\text{w}}}\) given that the ionization of water is an endothermic process.
(iv) State and explain the effect of increasing temperature on the pH of water.
(i) Deduce the acid and conjugate base ions that make up the phosphate buffer and state the ionic equation that represents the phosphate buffer.
(ii) Describe how the phosphate buffer minimizes the effect of the addition of a
strong base, \({\text{O}}{{\text{H}}^ - }{\text{(aq)}}\), to the buffer. Illustrate your answer with an ionic equation.
(iii) Describe how the phosphate buffer minimizes the effect of the addition of a
strong acid, \({{\text{H}}^ + }{\text{(aq)}}\), to the buffer. Illustrate your answer with an ionic equation.
(i) Explain why the pH of the ammonia solution is less than 13.
(ii) Estimate the pH at the equivalence point for the titration of hydrochloric acid with ammonia and explain your reasoning.
(iii) State the equation for the reaction of ammonia with water and write the \({K_{\text{b}}}\) expression for \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(aq)}}\).
(iv) When half the ammonia has been neutralized (the half-equivalence point), the pH of the solution is 9.25. Deduce the relationship between \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) and \({\text{[NH}}_4^ + {\text{]}}\) at the
half-equivalence point.
(v) Determine \({\text{p}}{K_{\text{b}}}\) and \({K_{\text{b}}}\) for ammonia based on the pH at the half-equivalence point.
(vi) Describe the significance of the half-equivalence point in terms of its effectiveness as a buffer.
Markscheme
(i) \({\text{(}}{K_{\text{w}}}{\text{)}} = {\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ - }{\text{] / (}}{K_{\text{w}}}{\text{)}} = {\text{[}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }{\text{][O}}{{\text{H}}^ - }{\text{]}}\);
Do not award mark if [ ] omitted or other brackets are used.
(ii) \({\text{[}}{{\text{H}}^ + }{\text{]}}\) increases, \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) decreases but still some present (\({K_{\text{w}}}\) constant) / \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) cannot go to zero as equilibrium present / \({\text{[O}}{{\text{H}}^ - }{\text{]}} = \frac{{{K_{\text{w}}}}}{{{\text{[}}{{\text{H}}^ + }{\text{]}}}}\), thus \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) cannot be zero / OWTTE;
Accept equilibrium present.
(iii) (changing T disturbs equilibrium) forward reaction favoured / equilibrium shifts to the right;
to use up (some of the) heat supplied;
\({{K_{\text{w}}}}\) increases (as both \({{\text{[}}{{\text{H}}^ + }{\text{]}}}\) and \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) increase);
(iv) (as \({{\text{[}}{{\text{H}}^ + }{\text{]}}}\) increases) pH decreases / \({\text{pH}} < 7\);
No mark for more acidic.
inverse relationship between pH and \({\text{[}}{{\text{H}}^ + }{\text{] / pH}} = - \log {\text{[}}{{\text{H}}^ + }{\text{] / pH}} = {\log _{10}}\frac{{\text{1}}}{{{\text{[}}{{\text{H}}^ + }{\text{]}}}}\);
Accept [H3O+] in place of [H+].
(i) Acid: \({{\text{H}}_2}{\text{PO}}_4^ - \);
(Conjugate) base: \({\text{HPO}}_4^{2 - }\);
No mark for NaH2PO4 or Na2HPO4.
\({{\text{H}}_2}{\text{PO}}_4^ - {\text{(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{HPO}}_4^{2 - }{\text{(aq)}}\);
Accept reverse equation or reaction with water.
Ignore state symbols, but equilibrium sign is required.
Accept OH– (ions) react with H+ (ions) to form H2O.
(ii) strong base/\({\text{O}}{{\text{H}}^ - }\) replaced by weak base (\({\text{HPO}}_4^{2 - }\), and effect minimized) / strong base reacts with acid of buffer / equilibrium in (i) shifts in forward direction;
Accept OH– added reacts with H+ to form H2O.
\({\text{O}}{{\text{H}}^ - }{\text{(aq)}} + {{\text{H}}_2}{\text{PO}}_4^ - {\text{(aq)}} \to {{\text{H}}_2}{\text{O(l)}} + {\text{HPO}}_4^{2 - }{\text{(aq)}}\);
Ignore state symbols, accept equilibrium sign.
(iii) strong acid/\({{\text{H}}^ + }\) replaced by weak acid (\({{\text{H}}_2}{\text{PO}}_4^ - \), and effect minimized) / strong acid reacts with base of buffer / equilibrium in (i) shifts in reverse direction;
\({{\text{H}}^ + }{\text{(aq)}} + {\text{HPO}}_4^{2 - }{\text{(aq)}} \to {{\text{H}}_2}{\text{PO}}_4^ - {\text{(aq)}}\);
Accept reaction with H3O+.
Ignore state symbols.
(i) \({\text{N}}{{\text{H}}_3}\) weak(er) base/partial dissociation;
\({\text{[O}}{{\text{H}}^ - }{\text{]}} < {\text{0.1(0)}}/{\text{pOH}} > 1{\text{ (thus pH}} < 13/{\text{pH}} + {\text{pOH}} = 14{\text{)}}\);
(ii) around \({\text{pH}} = 5\);
Accept a value between 4 and 6.
strong acid–weak base titration, (thus acidic) / at equivalence point, \({\text{NH}}_4^ + \) present is acidic / \({\text{NH}}_4^ + \rightleftharpoons {\text{N}}{{\text{H}}_3} + {{\text{H}}^ + }\);
(iii) \({\text{N}}{{\text{H}}_3}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{NH}}_4^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\);
Ignore state symbols, but equilibrium sign required.
\({K_{\text{b}}} = \frac{{{\text{[NH}}_4^ + {\text{][O}}{{\text{H}}^ - }{\text{]}}}}{{{\text{[N}}{{\text{H}}_3}{\text{]}}}}\);
(iv) \({\text{[N}}{{\text{H}}_3}{\text{]}} = {\text{[NH}}_4^ + {\text{]}}\);
(v) \({\text{pOH}} = 14.00 - 9.25 = 4.75\);
\({\text{p}}{K_{\text{b}}}{\text{ (}} = {\text{pOH)}} = 4.75\);
\({K_{\text{b}}} = 1.78 \times {10^{ - 5}}\);
Ignore units.
Award [3] for correct final answer.
(vi) optimum/most effective/highest buffer capacity/50%–50% buffer/equally effective as an acidic buffer and a basic buffer / OWTTE;
Examiners report
This was the second least commonly answered question. With the exception of the part on buffer chemistry where very few appreciated what was happening, the question was reasonably well done.
While many candidates gave the correct \({K_{\text{w}}}\) expression, it was not uncommon to either find the value of the constant or \({K_{\text{w}}} = {K_{\text{a}}} \times {K_{\text{b}}}\) given as the answers. A few included \({\text{[}}{{\text{H}}_{\text{2}}}{\text{O]}}\) in the expression. Candidates recognised that increasing the temperature shifts the equilibrium to the right, but most did not explain why, namely to use up some of the heat supplied.
Candidates generally concluded that formation of more \({{\text{H}}^ + }\) and \({\text{O}}{{\text{H}}^ - }\) ions gives a higher value of \({K_{\text{w}}}\). A significant number of candidates were able to state the effect of increasing temperature on the pH of water (it decreases) but failed to explain why. Some simply incorrectly stated that the pH would not change.
Many candidates gave the wrong formulas for the acid and the conjugate base ions of the buffer or offered \({\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{4}}}\) and \({\text{N}}{{\text{a}}_{\text{2}}}{\text{HP}}{{\text{O}}_{\text{4}}}\) as the answers. Some candidates gave good answers about the effect of adding a small amount of a strong acid or a strong base, but they could not write correct equations to show these two effects.
Nearly all candidates correctly said that the ammonia solution is a weak base because of partial dissociation and \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) would be less than 0.1 to give a pH less than 13. The majority of candidates correctly identified the pH around 4 – 6 because it is a titration between a strong acid and a weak base. When writing the equation for the reaction of ammonia and water some candidates did not write the equilibrium sign. The \({K_{\text{b}}}\) expression was correct in most cases. However, many did not recognise that at the half-equivalence point both the base and the conjugate acid concentrations are equal. The \({\text{p}}{K_{\text{b}}}\) and \({K_{\text{b}}}\) were correctly calculated from the pH of the solution by many candidates. However, most failed to realize that at the half-equivalence point the capacity of the buffer is optimum.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) \( \rightleftharpoons \) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.

Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.

Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, Kc.
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.

Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.

Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
Markscheme
molar mass of urea «4 \( \times \) 1.01 + 2 \( \times \) 14.01 + 12.01 + 16.00» = 60.07 «g mol-1»
«% nitrogen = \(\frac{{{\text{2}} \times {\text{14.01}}}}{{{\text{60.07}}}}\) \( \times \) 100 =» 46.65 «%»
Award [2] for correct final answer.
Award [1 max] for final answer not to two decimal places.
[2 marks]
«cost» increases AND lower N% «means higher cost of transportation per unit of nitrogen»
OR
«cost» increases AND inefficient/too much/about half mass not nitrogen
Accept other reasonable explanations.
Do not accept answers referring to safety/explosions.
[1 mark]

Note: Urea’s structure is more complex than that predicted from VSEPR theory.
[3 marks]
n(KNCO) «= 0.0500 dm3 \( \times \) 0.100 mol dm–3» = 5.00 \( \times \) 10–3 «mol»
«mass of urea = 5.00 \( \times \) 10–3 mol \( \times \) 60.07 g mol–1» = 0.300 «g»
Award [2] for correct final answer.
[2 marks]
\({K_{\text{c}}} = \frac{{[{{({{\text{H}}_2}{\text{N}})}_2}{\text{CO}}] \times [{{\text{H}}_2}{\text{O}}]}}{{{{[{\text{N}}{{\text{H}}_3}]}^2} \times [{\text{C}}{{\text{O}}_2}]}}\)
[1 mark]
«Kc» decreases AND reaction is exothermic
OR
«Kc» decreases AND ΔH is negative
OR
«Kc» decreases AND reverse/endothermic reaction is favoured
[1 mark]
ln K « = \(\frac{{ - \Delta {G^\Theta }}}{{RT}} = \frac{{ - 50 \times {{10}^3}{\text{ J}}}}{{8.31{\text{ J }}{{\text{K}}^{ - 1}}{\text{ mo}}{{\text{l}}^{ - 1}} \times 298{\text{ K}}}}\) » = –20
«Kc =» 2 \( \times \) 10–9
OR
1.69 \( \times \) 10–9
OR
10–9
Accept range of 20-20.2 for M1.
Award [2] for correct final answer.
[2 marks]
Any one of:
urea has greater molar mass
urea has greater electron density/greater London/dispersion
urea has more hydrogen bonding
urea is more polar/has greater dipole moment
Accept “urea has larger size/greater van der Waals forces”.
Do not accept “urea has greater intermolecular forces/IMF”.
[1 mark]

Award [1] for each correct interaction.
If lone pairs are shown on N or O, then the lone pair on N or one of the lone pairs on O MUST be involved in the H-bond.
Penalize solid line to represent H-bonding only once.
[2 marks]
2(H2N)2CO(s) + 3O2(g) → 4H2O(l) + 2CO2(g) + 2N2(g)
correct coefficients on LHS
correct coefficients on RHS
Accept (H2N)2CO(s) + \(\frac{3}{2}\)O2(g) → 2H2O(l) + CO2(g) + N2(g).
Accept any correct ratio.
[2 marks]
«V = \(\frac{{{\text{0.600 g}}}}{{{\text{60.07 g mo}}{{\text{l}}^{ - 1}}}}\) \( \times \) 22700 cm3 mol–1 =» 227 «cm3»
[1 mark]
lone/non-bonding electron pairs «on nitrogen/oxygen/ligand» given to/shared with metal ion
co-ordinate/dative/covalent bonds
[2 marks]
lone pairs on nitrogen atoms can be donated to/shared with C–N bond
OR
C–N bond partial double bond character
OR
delocalization «of electrons occurs across molecule»
OR
slight positive charge on C due to C=O polarity reduces C–N bond length
[1 mark]
60: CON2H4+
44: CONH2+
Accept “molecular ion”.
[2 marks]
3450 cm–1: N–H
1700 cm–1: C=O
Do not accept “O–H” for 3450 cm–1.
[2 marks]
1
[2 marks]
singlet
Accept “no splitting”.
[1 mark]
acts as internal standard
OR
acts as reference point
one strong signal
OR
12 H atoms in same environment
OR
signal is well away from other absorptions
Accept “inert” or “readily removed” or “non-toxic” for M1.
[2 marks]
Examiners report
Tin(II) chloride is a white solid that is commonly used as a reducing agent.
(i) State why you would expect tin(II) chloride to have a similar lattice enthalpy to strontium chloride, using section 9 of the data booklet.
(ii) Calculate the molar enthalpy change when strontium chloride is dissolved in water, using sections 18 and 20 of the data booklet.
(iii) Tin(II) chloride reacts with water to precipitate the insoluble basic chloride, Sn(OH)Cl.
Suggest why tin(II) chloride is usually dissolved in dilute hydrochloric acid.
Tin can also exist in the +4 oxidation state.
Vanadium can be reduced from an oxidation state of +4 to +3 according to the equation:
(i) Calculate the cell potential, EΘ, and the standard free energy, ΔGΘ, change for the reaction between the VO2+ and Sn2+ ions, using sections 1 and 2 of the data booklet.
EΘ:
ΔGΘ:
(ii) Deduce, giving your reason, whether a reaction between Sn2+(aq) and VO2+(aq) would be spontaneous.
Outline, giving the full electron configuration of the vanadium atom, what is meant by the term transition metal.
In an aqueous solution of vanadium(III) chloride, the vanadium exists as [V (H2O)6]3+, [VCl (H2O)5]2+ or [VCl2(H2O)4]+ depending on the concentration of chloride ions in the solution.
(i) Describe how Cl− and H2O bond to the vanadium ion.
(ii) Outline what would happen to the wavelength at which the vanadium complex ions would absorb light as the water molecules are gradually replaced by chloride ions, using section 15 of the data booklet.
Eight successive ionisation energies of vanadium are shown in the graph below:
(i) State the sub-levels from which each of the first four electrons are lost.
First: Second: Third: Fourth:
(ii) Outline why there is an increase in ionization energy from electron 3 to electron 5.
(iii) Explain why there is a large increase in the ionization energy between electrons 5 and 6.
(iv) Vanadium is comprised almost entirely of 51V. State the number of neutrons an atom of 51V has in its nucleus.
Markscheme
(i)
same charge AND same/similar ionic radius
(ii)
enthalpy of hydration «= −1483 + 2 (−359)» = −2201 «kJmol−1»
enthalpy of solution «= 2170 − 2201» = −31 «kJmol−1»
Award [2] for correct final answer.
Award [1 max] for +31 «kJmol−1».
Award [1 max] for ±4371.
(iii)
hydrochloric acid shifts equilibrium to left
OR
hydrochloric acid prevents the basic chloride forming/precipitating
Accept “hydrochloric acid reacts with «basic» chloride” OR “hydrochloric acid suppresses salt hydrolysis”.
(i)
EΘ «= 0.34 − 0.15» = 0.19«V»
∆GOΘ«= − nFEΘ = −2 × 96500 × 0.19» = −36670 / −37000«J» / − 37«kJ»
Accept −18335 «J» or −18 «kJ» as equation not specified.
(ii)
yes AND ∆GΘ is negative
OR
yes AND EΘ for the cell is positive
OR
yes AND Sn2+ (aq) is a stronger reducing agent than V3+(aq)
OR
yes AND EΘ SN4+ (aq) is more negative that EΘ or VO2+ (aq)
OR
yes AND VO2+ (aq) is a stronger oxidizing agent than Sn4+ (aq)
OR
yes AND EΘ for VO2+ (aq) is more positive than EΘ for SN4+ (aq)
Do not accept reference to anti-clockwise rule.
1s22s22p63s23p63d34s2
OR
1s22s22p63s23p64s23d3
incomplete d «sub-» level/orbital/shell «in its compounds/ions»
(i)
give/donate a lone/non-bonding electron pair
Accept “through the formation of a dative/ coordinate bond”.
Accept “by acting as Lewis bases”.
Do not accept “act as ligands”.
(ii)
«more chlorido ligands» smaller energy gap between split d-orbitals
OR
Cl− is lower than H2O in spectrochemical series
OR
Cl− is a weaker ligand/has lower charge density
the absorption will move to longer wavelengths
OR
the absorption wavelength will increase
Do not accept answers in terms of change of frequency.
(i)
First: 4s AND Second: 4s AND Third: 3d AND Fourth: 3d
Do not apply ECF from (c).
(ii)
«in the same sub-shell and a» decrease in electron-electron repulsion
OR
«in the same sub-shell and» as more electrons removed, the pull of of the nucleus/positive ions holds the remaining electrons more tightly
Do not accept “greater nuclear charge/ effective nuclear charge”.
(iii)
electron 5 is lost from the 3d orbital
OR
electron 5 is lost from the valence shell
electron 6 is lost from a 3p orbital
OR
electron 6 is lost from a «complete» inner shell
3p orbital/complete inner shell experiences a much larger effective nuclear charge
OR
3p orbital/complete inner shell is less well shielded
OR
3p orbital/complete inner shell is nearer the nucleus
Award [1 max] (for M1/M2) (ECF) if candidate recognises electrons 5 and 6 are from different levels.
(iv)
28
Examiners report
Phosgene, COCl2, is usually produced by the reaction between carbon monoxide and chlorine according to the equation:
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) At exactly 600°C the value of the equilibrium constant is 0.200. Calculate the standard Gibbs free energy change, , for the reaction, in kJ, using sections 1 and 2 of the data booklet. State your answer to three significant figures.
(iii) The standard enthalpy change of formation of phosgene, \(\Delta H_f^\Theta \), is −220.1kJmol−1. Determine the standard enthalpy change, \(\Delta H_{}^\Theta \), for the forward reaction of the equilibrium, in kJ, using section 12 of the data booklet.
(iv) Calculate the standard entropy change, \(\Delta S_{}^\Theta \), in JK−1, for the forward reaction at 25°C, using your answers to (a) (ii) and (a) (iii). (If you did not obtain an answer to (a) (ii) and/or (a) (iii) use values of +20.0 kJ and −120.0 kJ respectively, although these are not the correct answers.)
One important industrial use of phosgene is the production of polyurethanes. Phosgene is reacted with diamine X, derived from phenylamine.
(i) Classify diamine X as a primary, secondary or tertiary amine.
(ii) Phenylamine, C6H5NH2, is produced by the reduction of nitrobenzene, C6H5NO2. Suggest how this conversion can be carried out.
(iii) Nitrobenzene can be obtained by nitrating benzene using a mixture of concentrated nitric and sulfuric acids. Formulate the equation for the equilibrium established when these two acids are mixed.
(iv) Deduce the mechanism for the nitration of benzene, using curly arrows to indicate the movement of electron pairs.
The other monomer used in the production of polyurethane is compound Z shown below.
(i) State the name, applying IUPAC rules, of compound Z and the class of compounds to which it belongs.
Name:
Class:
(ii) Deduce the number of signals you would expect to find in the 1H NMR spectrum of compound Z, giving your reasons.
The mass spectrum and infrared (IR) spectrum of compound Z are shown below:
Mass spectrum
IR spectrum
(iii) Identify the species causing the large peak at m/z=31 in the mass spectrum.
(iv) Identify the bond that produces the peak labelled Q on the IR spectrum, using section 26 of the data booklet.
Phenylamine can act as a weak base. Calculate the pH of a 0.0100 mol dm−3 solution of phenylamine at 298K using section 21 of the data booklet.
Markscheme
(i)
\( \ll {K_{\rm{C}}}{\rm{ = }} \gg \frac{{\left[ {{\rm{COC}}{{\rm{l}}_{\rm{2}}}} \right]}}{{\left[ {{\rm{CO}}} \right]\left[ {{\rm{C}}{{\rm{l}}_2}} \right]}}\)
(ii)
T«= 600 + 273» = 873K
ΔGΘ = −8.31 × 873 × ln (0.200)
OR
ΔGΘ = « + » 11676 «J»
ΔGΘ = « + » 11.7 «kJ»
Accept 11.5 to 12.0.
Award final mark only if correct sig fig.
Award [3] for correct final answer.
(iii)
ΔHΘ = −220.1 − (−110.5)
ΔHΘ = −109.6 «kJ»
Award [2] for correct final answer.
Award [1] for −330.6, or +109.6 «kJ».
(iv)
ΔGΘ= −109.6 − (298 × ΔSΘ) = +11.7 «kJ»
ΔSΘ«\(\frac{{\left( {11.7 + 109.6} \right) \times {{10}^3}}}{{298}}\)»= −407 «JK−1»
Award [2] for correct final answer.
Award [2] for −470 «JK−1» (result from given values).
Do not penalize wrong value for T if already done in (a)(ii).
Award [1 max] for −0.407 «kJ K−1».
Award [1 max] for −138.9 «J K−1».
(i)
primary
(ii)
ALTERNATIVE 1:
«heat with» tin/Sn AND hydrochloric acid/HCl
aqueous alkali/OH–(aq)
ALTERNATIVE 2:
hydrogen/H2
nickel/Ni «catalyst»
Accept specific equations having correct reactants.
Do not accept LiAlH4 or NaBH4.
Accept Pt or Pd catalyst.
Accept equations having correct reactants.
(iii)
HNO3 + 2H2SO4 NO2+ + 2HSO4− + H3O+
Accept: HNO3 + H2SO4 NO2+ +HSO4− + H2O Accept HNO3 + H2SO4
H2NO3+ + HSO4− .
Accept equivalent two step reactions in which sulfuric acid first behaves as a strong acid and protonates the nitric acid, before behaving as a dehydrating agent removing water from it.
(iv)
curly arrow going from benzene ring to N of +NO2/NO2+
carbocation with correct formula and positive charge on ring
curly arrow going from C–H bond to benzene ring of cation
formation of organic product nitrobenzene AND H+
Accept mechanism with corresponding Kekulé structures.
Do not accept a circle in M2 or M3. Accept first arrow starting either inside the circle or on the circle.
M2 may be awarded from correct diagram for M3.
M4: Accept C6H5NO2 + H2SO4 if HSO4− used in M3.
(i)
Name: ethane-1,2-diol
Class: alcohol«s»
Accept ethan-1,2-diol / 1,2-ethanediol.
Do not accept “diol” for Class.
(ii)
two AND two hydrogen environments in the molecule
OR
two AND both CH2 and OH present
(iii)
+CH2OH
Accept CH3O+.
Accept [•CH2OH]+ and [•CH3O]+.
Do not accept answers in which the charge is missing.
(iv)
oxygen-hydrogen «bond»/O–H «in hydroxyl»
\({K_{\rm{b}}} \approx \frac{{{{\left[ {{\rm{O}}{{\rm{H}}^ - }} \right]}^2}}}{{\left[ {{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}{\rm{N}}{{\rm{H}}_{\rm{2}}}} \right]}} = {10^{ - 9.13}}/7.413 \times {10^{ - 10}}\)
\(\left[ {{\rm{O}}{{\rm{H}}^ - }} \right] = \sqrt {0.0100 \times {{10}^{ - 9.13}}} = 2.72 \times {10^{ - 6}}\)
\(\left[ {{{\rm{H}}^ + }} \right] = \frac{{1 \times {{10}^{ - 14}}}}{{2.72 \times {{10}^{ - 6}}}} = 3.67 \times {10^{ - 9}}\)
OR
pOH = 5.57
pH = −log [H+] = 8.44
Accept other approaches to the calculation.
Award [4] for correct final answer.
Accept any answer from 8.4 to 8.5.